Point Groups Continued Chapter 4
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proofs with Perpendicular Lines
3.4 Proofs with Perpendicular Lines EEssentialssential QQuestionuestion What conjectures can you make about perpendicular lines? Writing Conjectures Work with a partner. Fold a piece of paper D in half twice. Label points on the two creases, as shown. a. Write a conjecture about AB— and CD — . Justify your conjecture. b. Write a conjecture about AO— and OB — . AOB Justify your conjecture. C Exploring a Segment Bisector Work with a partner. Fold and crease a piece A of paper, as shown. Label the ends of the crease as A and B. a. Fold the paper again so that point A coincides with point B. Crease the paper on that fold. b. Unfold the paper and examine the four angles formed by the two creases. What can you conclude about the four angles? B Writing a Conjecture CONSTRUCTING Work with a partner. VIABLE a. Draw AB — , as shown. A ARGUMENTS b. Draw an arc with center A on each To be prof cient in math, side of AB — . Using the same compass you need to make setting, draw an arc with center B conjectures and build a on each side of AB— . Label the C O D logical progression of intersections of the arcs C and D. statements to explore the c. Draw CD — . Label its intersection truth of your conjectures. — with AB as O. Write a conjecture B about the resulting diagram. Justify your conjecture. CCommunicateommunicate YourYour AnswerAnswer 4. What conjectures can you make about perpendicular lines? 5. In Exploration 3, f nd AO and OB when AB = 4 units. -

Molecular Symmetry
Molecular Symmetry Symmetry helps us understand molecular structure, some chemical properties, and characteristics of physical properties (spectroscopy) – used with group theory to predict vibrational spectra for the identification of molecular shape, and as a tool for understanding electronic structure and bonding. Symmetrical : implies the species possesses a number of indistinguishable configurations. 1 Group Theory : mathematical treatment of symmetry. symmetry operation – an operation performed on an object which leaves it in a configuration that is indistinguishable from, and superimposable on, the original configuration. symmetry elements – the points, lines, or planes to which a symmetry operation is carried out. Element Operation Symbol Identity Identity E Symmetry plane Reflection in the plane σ Inversion center Inversion of a point x,y,z to -x,-y,-z i Proper axis Rotation by (360/n)° Cn 1. Rotation by (360/n)° Improper axis S 2. Reflection in plane perpendicular to rotation axis n Proper axes of rotation (C n) Rotation with respect to a line (axis of rotation). •Cn is a rotation of (360/n)°. •C2 = 180° rotation, C 3 = 120° rotation, C 4 = 90° rotation, C 5 = 72° rotation, C 6 = 60° rotation… •Each rotation brings you to an indistinguishable state from the original. However, rotation by 90° about the same axis does not give back the identical molecule. XeF 4 is square planar. Therefore H 2O does NOT possess It has four different C 2 axes. a C 4 symmetry axis. A C 4 axis out of the page is called the principle axis because it has the largest n . By convention, the principle axis is in the z-direction 2 3 Reflection through a planes of symmetry (mirror plane) If reflection of all parts of a molecule through a plane produced an indistinguishable configuration, the symmetry element is called a mirror plane or plane of symmetry . -

Chapter 1 – Symmetry of Molecules – P. 1
Chapter 1 – Symmetry of Molecules – p. 1 - 1. Symmetry of Molecules 1.1 Symmetry Elements · Symmetry operation: Operation that transforms a molecule to an equivalent position and orientation, i.e. after the operation every point of the molecule is coincident with an equivalent point. · Symmetry element: Geometrical entity (line, plane or point) which respect to which one or more symmetry operations can be carried out. In molecules there are only four types of symmetry elements or operations: · Mirror planes: reflection with respect to plane; notation: s · Center of inversion: inversion of all atom positions with respect to inversion center, notation i · Proper axis: Rotation by 2p/n with respect to the axis, notation Cn · Improper axis: Rotation by 2p/n with respect to the axis, followed by reflection with respect to plane, perpendicular to axis, notation Sn Formally, this classification can be further simplified by expressing the inversion i as an improper rotation S2 and the reflection s as an improper rotation S1. Thus, the only symmetry elements in molecules are Cn and Sn. Important: Successive execution of two symmetry operation corresponds to another symmetry operation of the molecule. In order to make this statement a general rule, we require one more symmetry operation, the identity E. (1.1: Symmetry elements in CH4, successive execution of symmetry operations) 1.2. Systematic classification by symmetry groups According to their inherent symmetry elements, molecules can be classified systematically in so called symmetry groups. We use the so-called Schönfliess notation to name the groups, Chapter 1 – Symmetry of Molecules – p. 2 - which is the usual notation for molecules. -

20. Geometry of the Circle (SC)
20. GEOMETRY OF THE CIRCLE PARTS OF THE CIRCLE Segments When we speak of a circle we may be referring to the plane figure itself or the boundary of the shape, called the circumference. In solving problems involving the circle, we must be familiar with several theorems. In order to understand these theorems, we review the names given to parts of a circle. Diameter and chord The region that is encompassed between an arc and a chord is called a segment. The region between the chord and the minor arc is called the minor segment. The region between the chord and the major arc is called the major segment. If the chord is a diameter, then both segments are equal and are called semi-circles. The straight line joining any two points on the circle is called a chord. Sectors A diameter is a chord that passes through the center of the circle. It is, therefore, the longest possible chord of a circle. In the diagram, O is the center of the circle, AB is a diameter and PQ is also a chord. Arcs The region that is enclosed by any two radii and an arc is called a sector. If the region is bounded by the two radii and a minor arc, then it is called the minor sector. www.faspassmaths.comIf the region is bounded by two radii and the major arc, it is called the major sector. An arc of a circle is the part of the circumference of the circle that is cut off by a chord. -
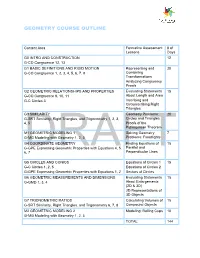
Geometry Course Outline
GEOMETRY COURSE OUTLINE Content Area Formative Assessment # of Lessons Days G0 INTRO AND CONSTRUCTION 12 G-CO Congruence 12, 13 G1 BASIC DEFINITIONS AND RIGID MOTION Representing and 20 G-CO Congruence 1, 2, 3, 4, 5, 6, 7, 8 Combining Transformations Analyzing Congruency Proofs G2 GEOMETRIC RELATIONSHIPS AND PROPERTIES Evaluating Statements 15 G-CO Congruence 9, 10, 11 About Length and Area G-C Circles 3 Inscribing and Circumscribing Right Triangles G3 SIMILARITY Geometry Problems: 20 G-SRT Similarity, Right Triangles, and Trigonometry 1, 2, 3, Circles and Triangles 4, 5 Proofs of the Pythagorean Theorem M1 GEOMETRIC MODELING 1 Solving Geometry 7 G-MG Modeling with Geometry 1, 2, 3 Problems: Floodlights G4 COORDINATE GEOMETRY Finding Equations of 15 G-GPE Expressing Geometric Properties with Equations 4, 5, Parallel and 6, 7 Perpendicular Lines G5 CIRCLES AND CONICS Equations of Circles 1 15 G-C Circles 1, 2, 5 Equations of Circles 2 G-GPE Expressing Geometric Properties with Equations 1, 2 Sectors of Circles G6 GEOMETRIC MEASUREMENTS AND DIMENSIONS Evaluating Statements 15 G-GMD 1, 3, 4 About Enlargements (2D & 3D) 2D Representations of 3D Objects G7 TRIONOMETRIC RATIOS Calculating Volumes of 15 G-SRT Similarity, Right Triangles, and Trigonometry 6, 7, 8 Compound Objects M2 GEOMETRIC MODELING 2 Modeling: Rolling Cups 10 G-MG Modeling with Geometry 1, 2, 3 TOTAL: 144 HIGH SCHOOL OVERVIEW Algebra 1 Geometry Algebra 2 A0 Introduction G0 Introduction and A0 Introduction Construction A1 Modeling With Functions G1 Basic Definitions and Rigid -

Symmetry of Graphs. Circles
Symmetry of graphs. Circles Symmetry of graphs. Circles 1 / 10 Today we will be interested in reflection across the x-axis, reflection across the y-axis and reflection across the origin. Reflection across y reflection across x reflection across (0; 0) Sends (x,y) to (-x,y) Sends (x,y) to (x,-y) Sends (x,y) to (-x,-y) Examples with Symmetry What is Symmetry? Take some geometrical object. It is called symmetric if some geometric move preserves it Symmetry of graphs. Circles 2 / 10 Reflection across y reflection across x reflection across (0; 0) Sends (x,y) to (-x,y) Sends (x,y) to (x,-y) Sends (x,y) to (-x,-y) Examples with Symmetry What is Symmetry? Take some geometrical object. It is called symmetric if some geometric move preserves it Today we will be interested in reflection across the x-axis, reflection across the y-axis and reflection across the origin. Symmetry of graphs. Circles 2 / 10 Sends (x,y) to (-x,y) Sends (x,y) to (x,-y) Sends (x,y) to (-x,-y) Examples with Symmetry What is Symmetry? Take some geometrical object. It is called symmetric if some geometric move preserves it Today we will be interested in reflection across the x-axis, reflection across the y-axis and reflection across the origin. Reflection across y reflection across x reflection across (0; 0) Symmetry of graphs. Circles 2 / 10 Sends (x,y) to (-x,y) Sends (x,y) to (x,-y) Sends (x,y) to (-x,-y) Examples with Symmetry What is Symmetry? Take some geometrical object. -
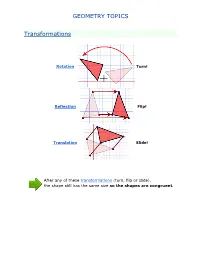
Geometry Topics
GEOMETRY TOPICS Transformations Rotation Turn! Reflection Flip! Translation Slide! After any of these transformations (turn, flip or slide), the shape still has the same size so the shapes are congruent. Rotations Rotation means turning around a center. The distance from the center to any point on the shape stays the same. The rotation has the same size as the original shape. Here a triangle is rotated around the point marked with a "+" Translations In Geometry, "Translation" simply means Moving. The translation has the same size of the original shape. To Translate a shape: Every point of the shape must move: the same distance in the same direction. Reflections A reflection is a flip over a line. In a Reflection every point is the same distance from a central line. The reflection has the same size as the original image. The central line is called the Mirror Line ... Mirror Lines can be in any direction. Reflection Symmetry Reflection Symmetry (sometimes called Line Symmetry or Mirror Symmetry) is easy to see, because one half is the reflection of the other half. Here my dog "Flame" has her face made perfectly symmetrical with a bit of photo magic. The white line down the center is the Line of Symmetry (also called the "Mirror Line") The reflection in this lake also has symmetry, but in this case: -The Line of Symmetry runs left-to-right (horizontally) -It is not perfect symmetry, because of the lake surface. Line of Symmetry The Line of Symmetry (also called the Mirror Line) can be in any direction. But there are four common directions, and they are named for the line they make on the standard XY graph. -

Symmetry in Chemistry - Group Theory
Symmetry in Chemistry - Group Theory Group Theory is one of the most powerful mathematical tools used in Quantum Chemistry and Spectroscopy. It allows the user to predict, interpret, rationalize, and often simplify complex theory and data. At its heart is the fact that the Set of Operations associated with the Symmetry Elements of a molecule constitute a mathematical set called a Group. This allows the application of the mathematical theorems associated with such groups to the Symmetry Operations. All Symmetry Operations associated with isolated molecules can be characterized as Rotations: k (a) Proper Rotations: Cn ; k = 1,......, n k When k = n, Cn = E, the Identity Operation n indicates a rotation of 360/n where n = 1,.... k (b) Improper Rotations: Sn , k = 1,....., n k When k = 1, n = 1 Sn = , Reflection Operation k When k = 1, n = 2 Sn = i , Inversion Operation In general practice we distinguish Five types of operation: (i) E, Identity Operation k (ii) Cn , Proper Rotation about an axis (iii) , Reflection through a plane (iv) i, Inversion through a center k (v) Sn , Rotation about an an axis followed by reflection through a plane perpendicular to that axis. Each of these Symmetry Operations is associated with a Symmetry Element which is a point, a line, or a plane about which the operation is performed such that the molecule's orientation and position before and after the operation are indistinguishable. The Symmetry Elements associated with a molecule are: (i) A Proper Axis of Rotation: Cn where n = 1,.... This implies n-fold rotational symmetry about the axis. -

CHAPTER 3 Parallel and Perpendicular Lines Chapter Outline
www.ck12.org CHAPTER 3 Parallel and Perpendicular Lines Chapter Outline 3.1 LINES AND ANGLES 3.2 PROPERTIES OF PARALLEL LINES 3.3 PROVING LINES PARALLEL 3.4 PROPERTIES OF PERPENDICULAR LINES 3.5 PARALLEL AND PERPENDICULAR LINES IN THE COORDINATE PLANE 3.6 THE DISTANCE FORMULA 3.7 CHAPTER 3REVIEW In this chapter, you will explore the different relationships formed by parallel and perpendicular lines and planes. Different angle relationships will also be explored and what happens to these angles when lines are parallel. You will continue to use proofs, to prove that lines are parallel or perpendicular. There will also be a review of equations of lines and slopes and how we show algebraically that lines are parallel and perpendicular. 114 www.ck12.org Chapter 3. Parallel and Perpendicular Lines 3.1 Lines and Angles Learning Objectives • Identify parallel lines, skew lines, and parallel planes. • Use the Parallel Line Postulate and the Perpendicular Line Postulate. • Identify angles made by transversals. Review Queue 1. What is the equation of a line with slope -2 and passes through the point (0, 3)? 2. What is the equation of the line that passes through (3, 2) and (5, -6). 3. Change 4x − 3y = 12 into slope-intercept form. = 1 = − 4. Are y 3 x and y 3x perpendicular? How do you know? Know What? A partial map of Washington DC is shown. The streets are designed on a grid system, where lettered streets, A through Z run east to west and numbered streets 1st to 30th run north to south. -

11.4 Arcs and Chords
Page 1 of 5 11.4 Arcs and Chords Goal By finding the perpendicular bisectors of two Use properties of chords of chords, an archaeologist can recreate a whole circles. plate from just one piece. This approach relies on Theorem 11.5, and is Key Words shown in Example 2. • congruent arcs p. 602 • perpendicular bisector p. 274 THEOREM 11.4 B Words If a diameter of a circle is perpendicular to a chord, then the diameter bisects the F chord and its arc. E G Symbols If BG&* ∏ FD&* , then DE&* c EF&* and DGs c GFs. D EXAMPLE 1 Find the Length of a Chord In ᭪C the diameter AF&* is perpendicular to BD&* . D Use the diagram to find the length of BD&* . 5 F C Solution E A Because AF&* is a diameter that is perpendicular to BD&* , you can use Theorem 11.4 to conclude that AF&* bisects B BD&* . So, BE ϭ ED ϭ 5. BD ϭ BE ϩ ED Segment Addition Postulate ϭ 5 ϩ 5 Substitute 5 for BE and ED . ϭ 10 Simplify. ANSWER ᮣ The length of BD&* is 10. Find the Length of a Segment 1. Find the length of JM&* . 2. Find the length of SR&* . H P 12 K C S C M N G 15 P J R 608 Chapter 11 Circles Page 2 of 5 THEOREM 11.5 Words If one chord is a perpendicular bisector M of another chord, then the first chord is a diameter. J P K Symbols If JK&* ∏ ML&* and MP&** c PL&* , then JK&* is a diameter. -

Geometry Course Outline Learning Targets Unit 1: Proof, Parallel, And
Geometry Course Outline Learning Targets Unit 1: Proof, Parallel, and Perpendicular Lines 1-1-1 Identify, describe, and name points, lines, line segments, rays and planes using correct notation. 1-1-2 Identify and name angles. 1-2-1 Describe angles and angle pairs. 1-2-2 Identify and name parts of a circle. 2-1-1 Make conjectures by applying inductive reasoning. 2-1-2 Recognize the limits of inductive reasoning. 2-2-1 Use deductive reasoning to prove that a conjecture is true. 2-2-2 Develop geometric and algebraic arguments based on deductive reasoning. 3-1-1 Distinguish between undefined and defined terms. 3-1-2 Use properties to complete algebraic two-column proofs. 3-2-1 Identify the hypothesis and conclusion of a conditional statement. 3-2-2 Give counterexamples for false conditional statements. 3-3-1 Write and determine the truth value of the converse, inverse, and contrapositive of a conditional statement. 3-3-2 Write and interpret biconditional statements. 4-1-1 Apply the Segment Addition Postulate to find lengths of segments. 4-1-2 Use the definition of midpoint to find lengths of segments. 4-2-1 Apply the Angle Addition Postulate to find angle measures. 4-2-2 Use the definition of angle bisector to find angle measures. 5-1-1 Derive the Distance Formula. 5-1-2 Use the Distance Formula to find the distance between two points on the coordinate plane. 5-2-1 Use inductive reasoning to determine the Midpoint Formula. 5-2-2 Use the Midpoint Formula to find the coordinates of the midpoint of a segment on the coordinate plane. -

Symmetry Groups
Symmetry Groups Symmetry plays an essential role in particle theory. If a theory is invariant under transformations by a symmetry group one obtains a conservation law and quantum numbers. For example, invariance under rotation in space leads to angular momentum conservation and to the quantum numbers j, mj for the total angular momentum. Gauge symmetries: These are local symmetries that act differently at each space-time point xµ = (t,x) . They create particularly stringent constraints on the structure of a theory. For example, gauge symmetry automatically determines the interaction between particles by introducing bosons that mediate the interaction. All current models of elementary particles incorporate gauge symmetry. (Details on p. 4) Groups: A group G consists of elements a , inverse elements a−1, a unit element 1, and a multiplication rule “ ⋅ ” with the properties: 1. If a,b are in G, then c = a ⋅ b is in G. 2. a ⋅ (b ⋅ c) = (a ⋅ b) ⋅ c 3. a ⋅ 1 = 1 ⋅ a = a 4. a ⋅ a−1 = a−1 ⋅ a = 1 The group is called Abelian if it obeys the additional commutation law: 5. a ⋅ b = b ⋅ a Product of Groups: The product group G×H of two groups G, H is defined by pairs of elements with the multiplication rule (g1,h1) ⋅ (g2,h2) = (g1 ⋅ g2 , h1 ⋅ h2) . A group is called simple if it cannot be decomposed into a product of two other groups. (Compare a prime number as analog.) If a group is not simple, consider the factor groups. Examples: n×n matrices of complex numbers with matrix multiplication.