(Canis Rufus) 5-Year Status Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Roots of Middle-Earth: William Morris's Influence Upon J. R. R. Tolkien
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2007 The Roots of Middle-Earth: William Morris's Influence upon J. R. R. Tolkien Kelvin Lee Massey University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Literature in English, British Isles Commons Recommended Citation Massey, Kelvin Lee, "The Roots of Middle-Earth: William Morris's Influence upon J. R. R. olkien.T " PhD diss., University of Tennessee, 2007. https://trace.tennessee.edu/utk_graddiss/238 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Kelvin Lee Massey entitled "The Roots of Middle-Earth: William Morris's Influence upon J. R. R. olkien.T " I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in English. David F. Goslee, Major Professor We have read this dissertation and recommend its acceptance: Thomas Heffernan, Michael Lofaro, Robert Bast Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) To the Graduate Council: I am submitting herewith a dissertation written by Kelvin Lee Massey entitled “The Roots of Middle-earth: William Morris’s Influence upon J. -

Afraid of Bear to Zuni: Surnames in English of Native American Origin Found Within
RAYNOR MEMORIAL LIBRARIES Indian origin names, were eventually shortened to one-word names, making a few indistinguishable from names of non-Indian origin. Name Categories: Personal and family names of Indian origin contrast markedly with names of non-Indian Afraid of Bear to Zuni: Surnames in origin. English of Native American Origin 1. Personal and family names from found within Marquette University Christian saints (e.g. Juan, Johnson): Archival Collections natives- rare; non-natives- common 2. Family names from jobs (e.g. Oftentimes names of Native Miller): natives- rare; non-natives- American origin are based on objects common with descriptive adjectives. The 3. Family names from places (e.g. following list, which is not Rivera): natives- rare; non-native- comprehensive, comprises common approximately 1,000 name variations in 4. Personal and family names from English found within the Marquette achievements, attributes, or incidents University archival collections. The relating to the person or an ancestor names originate from over 50 tribes (e.g. Shot with two arrows): natives- based in 15 states and Canada. Tribal yes; non-natives- yes affiliations and place of residence are 5. Personal and family names from noted. their clan or totem (e.g. White bear): natives- yes; non-natives- no History: In ancient times it was 6. Personal or family names from customary for children to be named at dreams and visions of the person or birth with a name relating to an animal an ancestor (e.g. Black elk): natives- or physical phenominon. Later males in yes; non-natives- no particular received names noting personal achievements, special Tribes/ Ethnic Groups: Names encounters, inspirations from dreams, or are expressed according to the following physical handicaps. -

Download Issue PDF the Spring 2020 Issue of Macalester Today Is Available for Viewing In
MACALESTER SPRING 2020 TODAY “The greatest privilege of my professional life” SEE PAGE 18 MACALESTER STAFF EDITOR SPRING 2020 Rebecca DeJarlais Ortiz ’06 TODAY [email protected] ART DIRECTION The ESC Plan / theESCplan.com CLASS NOTES EDITOR Robert Kerr ’92 PHOTOGRAPHER David J. Turner CONTRIBUTING WRITER Julie Hessler ’85 ASSISTANT VICE PRESIDENT FOR MARKETING AND COMMUNICATIONS Julie Hurbanis 14 18 26 32 36 CHAIR, BOARD OF TRUSTEES Jerry Crawford ’71 PRESIDENT FEATURES DEPARTMENTS Brian Rosenberg VICE PRESIDENT FOR ADVANCEMENT New Realities 10 Points of Struggle, Correspondence 2 D. Andrew Brown The COVID-19 pandemic forced Points of Pride 26 Household Words 3 ASSISTANT VICE PRESIDENT unprecedented, and often painful, Charting Macalester’s queer history FOR ENGAGEMENT decisions at Macalester this spring. over six decades 1600 Grand 4 Katie Ladas Macalester’s next president, Open LEFT TO RIGHT: PHOTO PROVIDED; DAVID J. TURNER; PHOTO PROVIDED; LISA HANEY, TRACEY BROWN TRACEY HANEY, LISA PROVIDED; TURNER; PHOTO J. DAVID PROVIDED; PHOTO RIGHT: TO LEFT MACALESTER TODAY (Volume 108, Number 2) “I Like to Dream Big” 14 Legends of the Night 32 Pantry, and the EcoHouse is published by Macalester College. It is mailed free of charge to alumni and friends Researcher and artist Jaye Sleep experts share their best of the college four times a year. Gardiner ’11 uses comics to advice—as well as some of their Class Notes 40 Circulation is 32,000. smash stereotypes and engage own sleep practice. You Said 41 TO UPDATE YOUR ADDRESS: new scientists. Email: [email protected] Waging Peace 36 Books 43 Call: 651-696-6295 or 1-888-242-9351 Write: Alumni Engagement Office, Macalester How to Practice Medicine Two alumni collaborate to support Weddings 45 College, 1600 Grand Ave., St. -

Twisted Trails of the Wold West by Matthew Baugh © 2006
Twisted Trails of the Wold West By Matthew Baugh © 2006 The Old West was an interesting place, and even more so in the Wold- Newton Universe. Until fairly recently only a few of the heroes and villains who inhabited the early western United States had been confirmed through crossover stories as existing in the WNU. Several comic book miniseries have done a lot to change this, and though there are some problems fitting each into the tapestry of the WNU, it has been worth the effort. Marvel Comics’ miniseries, Rawhide Kid: Slap Leather was a humorous storyline, parodying the Kid’s established image and lampooning westerns in general. It is best known for ‘outing’ the Kid as a homosexual. While that assertion remains an open issue with fans, it isn’t what causes the problems with incorporating the story into the WNU. What is of more concern are the blatant anachronisms and impossibilities the story offers. We can accept it, but only with the caveat that some of the details have been distorted for comic effect. When the Rawhide Kid is established as a character in the Wold-Newton Universe he provides links to a number of other western characters, both from the Marvel Universe and from classic western novels and movies. It draws in the Marvel Comics series’ Blaze of Glory, Apache Skies, and Sunset Riders as wall as DC Comics’ The Kents. As with most Marvel and DC characters there is the problem with bringing in the mammoth superhero continuities of the Marvel and DC universes, though this is not insurmountable. -

USBC Approved Bowling Balls
USBC Approved Bowling Balls (See rulebook, Chapter VII, "USBC Equipment Specifications" for any balls manufactured prior to January 1991.) ** Bowling balls manufactured only under 13 pounds. 5/17/2011 Brand Ball Name Date Approved 900 Global Awakening Jul-08 900 Global BAM Aug-07 900 Global Bank Jun-10 900 Global Bank Pearl Jan-11 900 Global Bounty Oct-08 900 Global Bounty Hunter Jun-09 900 Global Bounty Hunter Black Jan-10 900 Global Bounty Hunter Black/Purple Feb-10 900 Global Bounty Hunter Pearl Oct-09 900 Global Break Out Dec-09 900 Global Break Pearl Jan-08 900 Global Break Point Feb-09 900 Global Break Point Pearl Jun-09 900 Global Creature Aug-07 900 Global Creature Pearl Feb-08 900 Global Day Break Jun-09 900 Global DVA Open Aug-07 900 Global Earth Ball Aug-07 900 Global Favorite May-10 900 Global Head Hunter Jul-09 900 Global Hook Dark Blue/Light Blue Feb-11 900 Global Hook Purple/Orange Pearl Feb-11 900 Global Hook Red/Yellow Solid Feb-11 900 Global Integral Break Black Nov-10 900 Global Integral Break Rose/Orange Nov-10 900 Global Integral Break Rose/Silver Nov-10 900 Global Link Jul-08 900 Global Link Black/Red Feb-09 900 Global Link Purple/Blue Pearl Dec-08 900 Global Link Rose/White Feb-09 900 Global Longshot Jul-10 900 Global Lunatic Jun-09 900 Global Mach One Blackberry Pearl Sep-10 900 Global Mach One Rose/Purple Pearl Sep-10 900 Global Maniac Oct-08 900 Global Mark Roth Ball Jan-10 900 Global Missing Link Black/Red Jul-10 900 Global Missing Link Blackberry/Silver Jul-10 900 Global Missing Link Blue/White Jul-10 900 Global -

Holding the Wolf by the Ears: the Conservation of the Northern Rocky Mountain Wolf in Yellowstone National Park
Land & Water Law Review Volume 27 Issue 1 Article 2 1992 Holding the Wolf by the Ears: The Conservation of the Northern Rocky Mountain Wolf in Yellowstone National Park Timothy B. Strauch Follow this and additional works at: https://scholarship.law.uwyo.edu/land_water Recommended Citation Strauch, Timothy B. (1992) "Holding the Wolf by the Ears: The Conservation of the Northern Rocky Mountain Wolf in Yellowstone National Park," Land & Water Law Review: Vol. 27 : Iss. 1 , pp. 33 - 81. Available at: https://scholarship.law.uwyo.edu/land_water/vol27/iss1/2 This Article is brought to you for free and open access by Law Archive of Wyoming Scholarship. It has been accepted for inclusion in Land & Water Law Review by an authorized editor of Law Archive of Wyoming Scholarship. Strauch: Holding the Wolf by the Ears: The Conservation of the Northern Ro University of Wyoming College of Law LAND AND WATER LAW REVIEW VOLUME XXVII 1992 NUMBER 1 HOLDING THE WOLF BY THE EARS: THE CONSERVATION OF THE NORTHERN ROCKY MOUNTAIN WOLF IN YELLOWSTONE NATIONAL PARK Timothy B. Strauch* I. INTRODUCTION ....................................... 34 II. HISTORICAL PERSPECTIVE ON THE WOLF ................ 36 A. The Northern Rocky Mountain Wolf ............. 36 B. Early Reports and Killings of the Wolf in the Northern Rocky Mountain Area ................. 38 C. Wolf Population Decline Under Severe Predator Control Programs .............................. 40 D. The Wolf and Wolf Control in Yellowstone N ational P ark ................................. 42 III. WOLF RECOVERY AND CONSERVATION ................... 44 A. Current Status of the Northern Rocky Mountain W o lf ..... .. ....... .. ........ .. ... 4 4 B. The Endangered Species Act ................... 47 C. The Northern Rocky Mountain Wolf Recovery Plan 51 D. -

Undergraduate Student Yes Characterizing a Putative
Alex Abbott 41 Ouachita Baptist University Biology (BI) Undergraduate Student Yes Characterizing a Putative Mycobacteriophage Toxin-Antitoxin System Alex Abbott, Ruth Plymale Bacteria are under recurring threat of bacteriophage infection and possess defense systems to fight off these viruses. Surprisingly, these anti-phage defense systems may be encoded by prophage, bacteriophage DNA that has been integrated into a bacterial cell, thereby protecting the host bacterial cell from superinfection. Mycobacteriophage Xeno encodes a putative toxin-antitoxin (TA) system expected to defend against mycobacteriophage infection when Xeno is incorporated as a prophage in Mycobacterium smegmatis mc2155, forming the lysogen Mycobacterium smegmatis mc2155 (Xeno). The goal of this research is to identify which mycobacteriophage this putative TA system defends against, by screening more than 60 mycobacteriophage on M. smegmatis mc2155 and M. smegmatis mc2155(Xeno). Individual bacteriophage will be spotted on lawns of M. smegmatis mc2155 or M. smegmatis mc2155 (Xeno) and the number of plaQues will be counted and used to determine infection rate. If a phage produces 10x to 1000x more plaQues on M. smegmatis mc2155 than M. smegmatis mc2155 (Xeno), we will conclude that this phage is defended against by the TA system of the Xeno prophage. We will present the findings of our phage screening trials and our conclusions about the defensive range of the Xeno TA system. Joseph Akers 97 University of Central Arkansas Physics (PH) Undergraduate Student Yes Process Engineering: 3 Dimensional ColorSpace Correction Joseph Akers This project was developed to analyZe information pertaining to the color of an industrially manufactured product and provide corrective measures to achieve an ideal product. -
Crystal Reports Activex Designer
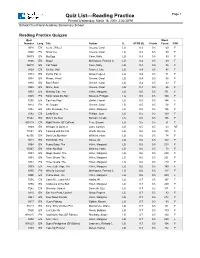
Quiz List—Reading Practice Page 1 Printed Wednesday, March 18, 2009 2:36:33PM School: Churchland Academy Elementary School Reading Practice Quizzes Quiz Word Number Lang. Title Author IL ATOS BL Points Count F/NF 9318 EN Ice Is...Whee! Greene, Carol LG 0.3 0.5 59 F 9340 EN Snow Joe Greene, Carol LG 0.3 0.5 59 F 36573 EN Big Egg Coxe, Molly LG 0.4 0.5 99 F 9306 EN Bugs! McKissack, Patricia C. LG 0.4 0.5 69 F 86010 EN Cat Traps Coxe, Molly LG 0.4 0.5 95 F 9329 EN Oh No, Otis! Frankel, Julie LG 0.4 0.5 97 F 9333 EN Pet for Pat, A Snow, Pegeen LG 0.4 0.5 71 F 9334 EN Please, Wind? Greene, Carol LG 0.4 0.5 55 F 9336 EN Rain! Rain! Greene, Carol LG 0.4 0.5 63 F 9338 EN Shine, Sun! Greene, Carol LG 0.4 0.5 66 F 9353 EN Birthday Car, The Hillert, Margaret LG 0.5 0.5 171 F 9305 EN Bonk! Goes the Ball Stevens, Philippa LG 0.5 0.5 100 F 7255 EN Can You Play? Ziefert, Harriet LG 0.5 0.5 144 F 9314 EN Hi, Clouds Greene, Carol LG 0.5 0.5 58 F 9382 EN Little Runaway, The Hillert, Margaret LG 0.5 0.5 196 F 7282 EN Lucky Bear Phillips, Joan LG 0.5 0.5 150 F 31542 EN Mine's the Best Bonsall, Crosby LG 0.5 0.5 106 F 901618 EN Night Watch (SF Edition) Fear, Sharon LG 0.5 0.5 51 F 9349 EN Whisper Is Quiet, A Lunn, Carolyn LG 0.5 0.5 63 NF 74854 EN Cooking with the Cat Worth, Bonnie LG 0.6 0.5 135 F 42150 EN Don't Cut My Hair! Wilhelm, Hans LG 0.6 0.5 74 F 9018 EN Foot Book, The Seuss, Dr. -

TEXAS ENVIRONMENTAL LAW JOURNAL Volume 50 Winter 2020 Number 2
TEXAS ENVIRONMENTAL LAW JOURNAL Volume 50 Winter 2020 Number 2 ARTICLES PUBLIC DRINKING WATER SYSTEMS: WHAT EVERY ENVIRONMENTAL LAWYER SHOULD KNOW Kellie E. Billings-Ray 233 RED WOLF COALITION V. UNITED STATES FISH AND WILDLIFE SERVICE: RED SCARE Edward Fitzgerald 247 COMPARING COMPACTS: EXAMINING THE SUPREME COURT’S DECISION IN MONTANA V. WYOMING TO EVALUATE TEXAS V. NEW MEXICO REGARDING THE RIO GRANDE COMPACT Eugene C. White 291 NOTES WATER ALLOCATION LAWS AND ENERGY PRODUCTION ACROSS THE UNITED STATES: HOW TEXAS’S UNIQUE LAWS AND POSITION AS AN ENERGY POWERHOUSE PUT IT AT AN UNFAIR ADVANTAGE Zoe W. Oldham 307 THE FINAL STRAW?: EVALUATING POSSIBLE CHALLENGES TO SINGLE-USE PLASTIC STRAW BANS Dayna Smith 331 BLOCKCHAIN FOR CLEAN ENERGY – A “DISTRIBUTED” APPROACH TO SAVING THE PLANET Benjamin R. Zeter 353 DEVELOPMENTS AIR – John Turney, Jacob Gildan 377 FEDERAL UPDATE – Amanda G. Halter, Meredith Luneack 381 NATURAL RESOURCES – Patrick Leahy, Neha Singh 387 PUBLICATIONS – Joshua D. Katz, Emily Meier 391 STATE CASENOTE – Stacie M. Dowell, Thomas Kagerer 393 WATER RIGHTS & UTILITIES – Emily Rogers, Kimberly Kelley, Patrick Maloney 399 STATE BAR SECTION NEWS Prepared through The University of Texas School of Law Publications Office ISSN 0163-545X Copyright © 2020 Environmental and Natural Resources Section of the State Bar of Texas and The University of Texas School of Law Texas Environmental Law Journal Please cite as: TEX. ENVTL. L. J. TEXAS ENVIRONMENTAL LAW JOURNAL Volume 50 Winter 2020 Number 2 S TATE B AR OF T EXAS ENVIRONMENTAL AND NATURAL RESOURCES LAW SECTION P.O. Box 220, Mailstop S-520 Austin, Texas 78767-0220 www.texenrls.org EDITORIAL BOARD Editor-In-Chief Assistant Editor for Production Ashleigh K. -

A Comprehensive Review and Evaluation of the Red Wolf (Canis Rufus) Recovery Program
A Comprehensive Review and Evaluation of the Red Wolf (Canis rufus) Recovery Program Final Report - 11/14/2014 Wildlife Management Institute, Inc. 2014 Executive Summary The United States Fish and Wildlife Service (FWS) contracted with the Wildlife Management Institute (WMI) to conduct an independent review and evaluation of the red wolf (Canis rufus) recovery program. At the direction of the FWS, the review focused on numerous questions with respect to three elements of the recovery program: supporting science, program management, and human dimensions. WMI reviewed more than 200 documents received from the FWS, interviewed 20 FWS employees and 4 North Carolina Wildlife Resources Commission (NCWRC) staff at various management levels, commissioned literature reviews of red wolf genetics and ecology, conducted 2 public meetings in the red wolf restoration area, and conducted public opinion surveys. Our report was reviewed by outside experts in wolf ecology and management. This report is an evaluation and synthesis of the available scientific literature, reports, documents, interviews with FWS staff, and public comments received during the review period. The report is not intended, nor should it be construed, to be a decision document with recommendations relative to the fate of the current red wolf recovery program. The report represents the views of the authors and not necessarily those of the FWS. Supporting Science The experimental release of captive red wolves to the wild in 1987 proved red wolves could survive and successfully reproduce in the wild. The FWS staff directly involved with the red wolf recovery program has a thorough understanding of the current science regarding red wolf ecology in the area. -

The Starman Omnibus Vol. 2 PDF Book
THE STARMAN OMNIBUS VOL. 2 PDF, EPUB, EBOOK James Robinson | 416 pages | 11 Sep 2012 | DC Comics | 9781401221959 | English | New York, NY, United States The Starman Omnibus Vol. 2 PDF Book Martin Luther King Jr. Collects Starman 2nd Series and 1,,, Stars and S. It's stuck somewhere between a character-driven indie comic like Strangers In Paradise and a classic DC superhero comic. Fate, the Shade, and the Golden Age Sandman. In the pages of Starman we get a looser, more cartoony style from Harris, but with hint at the refined style he currently draws in. This made me realize that I am not a huge fan of Tony Harris's art. All rights reserved. Zero Hour: Crisis in Time 25th Anniversary. However, he got stranded on Earth, the Kingdom Come universe, thus witnessing the dramatic events on that Earth and receiving added damage to his frail mind, worsened by the lack of the advanced medication to which he had had access in his own time. Of course there is his wise mentor in his father, Ted. Books by James Robinson. Help Learn to edit Community portal Recent changes Upload file. Creepy Eerie. From Wikipedia, the free encyclopedia. Much like Opal City itself, Robinson crafts a fantastic blend of old and new in his story. He pickpocketed a pistol and fired on the group. An old guy now, but his thought process is shown here, and his arc striked curiosity inside of me: now I want to know more about the character, and how he was when younger This book gave me a bunch of mixed feelings. -

The Comics & Graphic Novel Bulletin Of
MEANWHILE Kirby Robert E. Howard Frank Miller Barry Windsor Smith Kirby Ster- Neal Adams anko Pre- Tom Palmer Raphaelites. Roy Thomas Harlan Ellison John Buscema Javier Rodriguez Mark Waid The Comics & Graphic Novel Bulletin of Fantastic Four: Grand Design builds The 1960s were an amaz- in the first volume of Marvel on the success of Marvel’s previous ing time to be a kid. Satur- Comics Mini-Books Collecti- Grand Design trilogy dedicated to the day morning cartoons, ble Box Set: A History and Facsimiles of Marvel’s X-Men (see 741.5 #18). Published in comic books everywhere, the Oversize “Treasury” format, Vol- Smallest Comic Books (Harry and all the gimmicks sold ume 1 covers the history of the Cos- N. Abrams). Published in mic Quartet from their initial outings at the candy store and 1966, these 5/8” x 7/8” against the Mole Man, Doctor Doom gumball machines: Mars comics were smaller than a and the Skrulls up to the celestial Attacks cards, Big Daddy postage stamp and hardly bigger than the penny they showdowns of the 1980s. Produced Roth Weird-o stickers, cost. They featured several of with the “aged” look of old comics, and...Marvel Comics Mini- FF:GD includes developmental mate- the headliners who made rials and a reprint of a FF classic! books! If you’ve never that decade the Marvel Age experienced or even heard of Comics: the Amazing Spi- of these, that’s okay. They der-Man, Captain America, BELOW: Examples of the the Mighty Thor, the Incredi- Marvel mini-books print- were mostly distributed ble Hulk, WW2 super-soldier ed at gumball size.