Moyamoya Disease: a Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Moyanioya Syndrome*
Rey. Argent. Neuroc. 2005; 19: 31 Actualización MOYANIOYA SYNDROME* Edward R. Smith, MD, R. Michael Scott, MD Department of Neurosurgery, The Children's Hospital, Boston RESUMEN El síndrome de Moyamoya es una vasculopatía caracterizada por la estenosis crónica y progresiva de la arteria carótida interna intracraneal. El diagnóstico se realiza en base a hallazgos clínicos y radiológicos que incluyen la muy característica estenosis de la carótida interna con un importante desarrollo de circulación colateral. Los pacientes adultos con Moyamoya con frecuencia se presentan con cuadros hemorrágicos. En cambio, los niños usualmente presentan ataques isquémicos transitorios (AIT) o infartos isquémicos y en ellos es frecuente un diagnóstico más tardío del síndrome Moyamoya. La progresión de la enfermedad puede ser lenta con eventos intermitentes o puede ser fulminante, con un rápido deterioro neurológico. Cualquiera sea laforma de evolucionar, el síndrome de Moyamoya suele presentar un empeoramiento clínico y radiológico progresivo en los pacientes no tratados. La cirugía es recomendada para el tratamiento de pacientes con eventos isquémicos cerebrales recurrentes o progresivos que presentan una reducción de la reserva de perfusión cerebral. Múltiples técnicas operatorias han sido descriptas con el propósito de prevenir injurias cerebrales isquémicas mediante un aumento del flujo sanguíneo colateral en áreas corticales hipoperfundidas utilizando la circulación carotídea externa como vascularización donante. Este trabajo discute las diferentes formas terapéuticas con un énfasis especial en la utilización de la sinangiosis pial como método de revascularización indirecta. La utilización de la sinangiosis pial es unaforma de revascularización cerebral efectiva y durable en el síndrome de Moyamoya y debe ser considerada como una forma primaria de tratamiento de esta entidad, especialmente en la población infantil. -

Fibromyalgia and Chronic Fatigue
Fibromyalgia and Chronic Fatigue Paul G. Swingle, Ph.D., R. Psych. My previous radio program and my present webcast program are both entitled “It’s all in your head!” How I came up with this title was listening to the discouraged patients that I routinely treated, and still treat, who have been told, by the doctors they have consulted, that the pain, discomfort, distress, debilitating fatigue, sleep disturbance and depression that they endure is “all in your head.” What this remark is meant to imply, of course, is that there is no physical cause of the condition and that the symptoms are manifestations of psychological factors. My response to these patients is that “of course it is in your head, where else would it be?” My remark however is not disparaging but rather indicates the actual location of many of the patients symptoms. The brain controls everything so all symptoms are associated with brain activity. The remarkable effectiveness of neurotherapy for the treatment of the symptoms associated with fibromyalgia and chronic fatigue is due to the fact that the brain functioning associated with the symptoms is treated. Although this derogatory sentiment about fibromyalgia patients was widespread among health care providers in the past, it is remarkable to me that it still persists with many doctors to this day. A few days ago I was surfing the internet health news feeds and was dismayed to find an item entitled “Fibromyalgia: A mental illness?” In this item was a quote from a Canadian neurologist stating that fibromyalgia was a term waiting for a definition. -

Complete Issue (PDF)
JUNE 2018 AJNR VOLUME 39 • PP 993–1191 JUNE 2018 THE JOURNAL OF DIAGNOSTIC AND VOLUME 39 INTERVENTIONAL NEURORADIOLOGY NUMBER 6 WWW.AJNR.ORG Multisite concordance of DSC for brain tumors Comparison of 3T intracranial vessel wall sequences Ophthalmic artery collaterals in Moyamoya disease Official Journal ASNR • ASFNR • ASHNR • ASPNR • ASSR Low-profile Visualized Intraluminal Support Stent Deployment. Refined. Braided Coil Assist Stents with High Neck Coverage, Excellent Visibility and Improved Conformability* Aneurysm Therapy Solutions For more information or a product demonstration, contact your local MicroVention representative: MicroVention Worldwide Innovation Center PH +1.714.247.8000 35 Enterprise *Humanitarian Device: Authorized by Federal Law for use with bare platinum Aliso Viejo, CA 92656 USA embolic coils for the treatment of unruptured, wide neck (neck ≥ 4 mm or dome to neck ratio < 2), intracranial, saccular aneurysms arising from a parent MicroVention UK Limited PH +44 (0) 191 258 6777 vessel with a diameter ≥ 2.5 mm and ≤ 4.5 mm. The effectiveness of this device MicroVention Europe, S.A.R.L. PH +33 (1) 39 21 77 46 for this use has not been demonstrated. MicroVention Deutschland GmbH PH +49 211 210 798-0 microvention.com MICROVENTION and LVIS are registered trademarks of MicroVention, Inc. • Refer to Instructions for Use, contraindications and warnings for additional information. Federal (USA) law restricts this device for sale by or on the order of a physician. ©2018 MicroVention, Inc. Jan. 2018 Now you have 24 hours to make a lifetime of difference in stroke patients like Nora The Trevo Retriever is the only device cleared to reduce disability in stroke patients up to 24 hours from time last seen well. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Vascular Dementia: an Overview of Current Challenges and Gaps
Stroke and Dementia: what research tells us UK Stroke Assembly 2016 JM Wardlaw University of Edinburgh NHS Lothian www.strokeassembly.org.uk #strokeassembly Definitions Stroke Dementia Sudden onset focal A syndrome where there neurological deficit which is deterioration in last more than 24 hours memory, thinking, attributable to a vascular behaviour such that the defect and has no other person is no longer able identifiable cause to perform everyday activities by themselves www.strokeassembly.org.uk #strokeassembly Stroke – the importance • Stroke, 16.9million new strokes per year, – A third are disabled and dependent – 2nd commonest cause of death – Commonest cause of dependency in adults Infarct, or ischaemic stroke Haemorrhagic stroke www.strokeassembly.org.uk #strokeassembly Cerebrovascular disease – the importance • ‘Silent’ cerebrovascular disease, even bigger issue – Up to 45% of 35.6million dementias – Cognitive impairment, mobility problems Normal White matter disease www.strokeassembly.org.uk #strokeassembly Dementia • 47.5 million people have dementia world wide • 7.7million new cases per year • Affects different people in different ways • Major cause of dependency and disability amongst older people • Alzheimer’s disease commonest cause – 60-70% of cases • Vascular dementia 2nd commonest – 25-45% of all cases www.strokeassembly.org.uk #strokeassembly Dementia: historical perspective 1800’s – Blood vessel diseases 1900’s – Alzheimers disease - dominant 1970’s – ‘Multi-infarct dementia’ – vascular 1990’s–2000’s - ‘Vascular -

TIA Vs CVA (STROKE)
Phone: 973.334.3443 Email: [email protected] NJPR.com TIA vs CVA (STROKE) What is the difference between a TIA and a stroke? Difference Between TIA and Stroke • Both TIA and stroke are due to poor blood supply to the brain. • Stroke is a medical emergency and it’s a life-threatening condition. • The symptoms of TIA and Stroke may be same but TIA symptoms will recover within 24 hours. TRANSIENT ISCHEMIC ATTACK ● Also known as: TIA, mini stroke 80 E. Ridgewood Avenue, 4th Floor Paramus, NJ 07652 TIA Causes ● A transient ischemic attack has the same origins as that of an ischemic stroke, the most common type of stroke. In an ischemic stroke, a clot blocks the blood supply to part of your brain. In a transient ischemic attack, unlike a stroke, the blockage is brief, and there is no permanent damage. ● The underlying cause of a TIA often is a buildup of cholesterol- containing fatty deposits called plaques (atherosclerosis) in an artery or one of its branches that supplies oxygen and nutrients to your brain. ● Plaques can decrease the blood flow through an artery or lead to the development of a clot. A blood clot moving to an artery that supplies your brain from another part of your body, most commonly from your heart, also may cause a TIA. CEREBROVASCULAR ACCIDENT/STROKE Page 2 When the brain’s blood supply is insufficient, a stroke occurs. Stroke symptoms (for example, slurring of speech or loss of function in an arm or leg) indicate a medical emergency. Without treatment, the brain cells quickly become impaired or die. -

Characteristics of Moyamoya Disease in the Older Population: Is It Possible to Define a Typical Presentation and Optimal Therapeutical Management?
Journal of Clinical Medicine Review Characteristics of Moyamoya Disease in the Older Population: Is It Possible to Define a Typical Presentation and Optimal Therapeutical Management? Ignazio G. Vetrano 1,* , Anna Bersano 2 , Isabella Canavero 2, Francesco Restelli 1, Gabriella Raccuia 1, Elisa F. Ciceri 3, Giuseppe Faragò 3, Andrea Gioppo 3, Morgan Broggi 1 , Marco Schiariti 1, Laura Gatti 4 , Paolo Ferroli 1 and Francesco Acerbi 1,5 1 Department of Neurosurgery, Fondazione IRCCS Istituto Neurologico Carlo Besta, 20133 Milan, Italy; [email protected] (F.R.); [email protected] (G.R.); [email protected] (M.B.); [email protected] (M.S.); [email protected] (P.F.); [email protected] (F.A.) 2 Cerebrovascular Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, 20133 Milan, Italy; [email protected] (A.B.); [email protected] (I.C.) 3 Interventional Neuroradiology Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, 20133 Milan, Italy; [email protected] (E.F.C.); [email protected] (G.F.); [email protected] (A.G.) 4 Cellular Neurobiology Laboratory, Fondazione IRCCS Istituto Neurologico Carlo Besta, 20133 Milan, Italy; [email protected] 5 Experimental Microsurgical Laboratory, Fondazione IRCCS Istituto Neurologico Carlo Besta, Citation: Vetrano, I.G.; Bersano, A.; 20133 Milan, Italy Canavero, I.; Restelli, F.; Raccuia, G.; * Correspondence: [email protected] Ciceri, E.F.; Faragò, G.; Gioppo, A.; Broggi, M.; Schiariti, M.; et al. Abstract: Whereas several studies have been so far presented about the surgical outcomes in terms of Characteristics of Moyamoya Disease mortality and perioperative complications for elderly patients submitted to neurosurgical treatments, in the Older Population: Is It Possible the management of elderly moyamoya patients is unclear. -
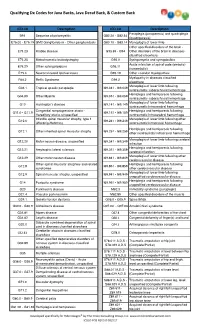
ICD-10 Backs
Qualifying Dx Codes for Java Backs, Java Decaf Back, & Custom Back ICD-10 Description ICD-10 Description Paraplegia (paraparesis) and quadriplegia B91 Sequelae of poliomyelitis G82.20 - G82.54 (quadriparesis) E75.00 - E75.19 GM2 Gangliosidosis - Other gangliosidosis G83.10 - G83.14 Monoplegia of lower limb Other specified disorders of the brain - E75.23 Krabbe disease G93.89 - G94 Other disorders of the brain in diseases classified elsewhere E75.25 Metachromatic leukodystrophy G95.0 Syringomyelia and syringobulbia Acute infarction of spinal code (embolic) E75.29 Other sphingolipidosis G95.11 (nonembolic) E75.4 Neuronal ceroid lipofuscinosis G95.19 Other vascular myelopathies Myelopathy in diseases classified F84.2 Rett's Syndrome G99.2 elsewhere Monoplegia of lower limb following G04.1 Tropical spastic paraplegia I69.041 - I69.049 nontraumatic subarachnoid hemorrhage Hemiplegia and hemiparesis following G04.89 Other Myelitis I69.051 - I69.059 nontraumatic subarachnoid hemorrhage Monoplegia of lower limb following G10 Huntington's disease I69.141 - I69.149 nontraumatic intracerebral hemorrhage Congenital nonprogressive ataxia - Hemiplegia and hemiparesis following G11.0 - G11.9 I69.151 - I69.159 Hereditary ataxia, unspecified nontraumatic intracerebral hemorrhage Infantile spinal muscular atrophy, type 1 Monoplegia of lower limb following other G12.0 I69.241 - I69.249 (Werdnig-Hoffman) nontraumatic intracranial hemorrhage Hemiplegia and hemiparesis following G12.1 Other inherited spinal muscular atrophy I69.251 - I69.259 other nontraumatic -

The Secretion of Oxygen Into the Swim-Bladder of Fish III
CORE Metadata, citation and similar papers at core.ac.uk Provided by PubMed Central The Secretion of Oxygen into the Swim-Bladder of Fish III. The role of carbon dioxide JONATHAN B. WITTENBERG, MARY J. SCHWEND, and BEATRICE A. WITTENBERG From the Department of Physiology, Albert Einstein College of Medicine, Yeshiva University, New York, and the Marine Biological Laboratory, Woods Hole, Massachusetts ABSTRACT The secretion of carbon dioxide accompanying the secretion of oxygen into the swim-bladder of the bluefish is examined in order to distinguish among several theories which have been proposed to describe the operation of the rete mirabile, a vascular countercurrent exchange organ. Carbon dioxide may comprise 27 per cent of the gas secreted, corresponding to a partial pres- sure of 275 mm Hg. This is greater than the partial pressure that would be generated by acidifying arterial blood (about 55 mm Hg). The rate of secretion is very much greater than the probable rate of metabolic formation of carbon dioxide in the gas-secreting complex. It is approximately equivalent to the probable rate of glycolytic generation of lactic acid in the gas gland. It is con- cluded that carbon dioxide brought into the swim-bladder is liberated from blood by the addition of lactic acid. The rete mirabile must act to multiply the primary partial pressure of carbon dioxide produced by acidification of the blood. The function of the rete mirabile as a countercutrent multiplier has been proposed by Kuhn, W., Ramel, A., Kuhn, H. J., and Marti, E., Experientia, 1963, 19, 497. Our findings provide strong support for their theory. -

Central Pain: Clinical and Physiological J Neurol Neurosurg Psychiatry: First Published As 10.1136/Jnnp.61.1.62 on 1 July 1996
626Journal ofNeurology, Neurosurgery, and Psychiatry 1996;61:62-69 Central pain: clinical and physiological J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.61.1.62 on 1 July 1996. Downloaded from characteristics David Bowsher Abstract spinothalamic pathway, its relays, or projec- Objectives-To study the clinical and tions may cause central pain,' and modern pathophysiological features of central radiological techniques have tended to con- pain due to damage to the CNS. firm this.9 '" Because of this, and of recent Methods-156 patients (mostly with considerations on pathophysiology,'5 '" the ischaemic strokes, some with infarct after condition is now known as "central poststroke subarachnoid haemorrhage and other pain (CPSP)"'.'7 Stroke is not the only condi- cerebral conditions; one with bulbar and tion causing such central pains of cerebral ori- others with spinal pathology) with central gin.' lxII 2' Furthermore, identical pains occur pain have been investigated clinically and after some spinal lesions.- 22 varying numbers instrumentally with respect to quantitative somatosensory perception thresholds and autonomic Patients function. Between 1983 and 1993, 156 patients have Results-Pain onset was immediate in a been referred to me, in whom a diagnosis of minority; and from a week or two up to central pain due to a cerebral or spinal lesion six years in > 60%. For those with supra- has been made. Of these, 112 (64 (57)%'Y spinal ischaemic lesions, the median age male) had a history of a stroke episode (cere- of onset was 59; dominant and non- brovascular accident, CVA patients), includ- dominant sides were equally affected. -

A Case Report of Moyamoya Disease Presenting As Headache in a 35-Year-Old Hispanic Man
Open Access Case Report DOI: 10.7759/cureus.4426 A Case Report of Moyamoya Disease Presenting as Headache in a 35-year-old Hispanic Man Daniel B. Azzam 1 , Ajay N. Sharma 2 , Ekaterina Tiourin 3 , Alvin Y. Chan 1 1. Neurosurgery, University of California, Irvine, USA 2. Dermatology, University of California, Irvine, USA 3. Miscellaneous, University of California, Irvine, USA Corresponding author: Daniel B. Azzam, [email protected] Abstract Moyamoya disease (MMD) is a rare, chronic vaso-occlusive disease affecting the arteries of the Circle of Willis, leading to the development of characteristic collateral vessels. In this paper, we present a case of a 35-year-old Hispanic male who presented to the emergency department with new onset headaches. On examination, Glasgow Coma Scale score was 3T. The patient was investigated with head CT scan and cerebral angiogram, diagnosed as MMD, and treated with emergent ventriculostomy. Ultimately, the patient underwent extracranial-intracranial (EC-IC) bypass surgery for treatment of Moyamoya. Categories: Emergency Medicine, Neurology, Neurosurgery Keywords: moyamoya, headache, hemorrhage, stroke, neurosurgery Introduction Moyamoya disease (MMD) is a progressive cerebrovascular disorder characterized by progressive stenosis of the terminal portions of the intracranial internal carotid arteries due to hypertrophy of smooth muscle in the vessel walls [1-4]. Reduced blood flow to the brain leads to the growth of collateral vasculature such as branches from small leptomeningeal vessels. Imaging of the collateral vasculature was characterized in Japan as a “puff of smoke,” or moyamoya in Japanese. Incidence of MMD is the highest in Japan, where there are three cases per 100,000, and in females (2:1 ratio) [2]. -

Genetics of Amyotrophic Lateral Sclerosis
GENETICS VIGNETTE Genetics of Amyotrophic Lateral Sclerosis J. Gorodenker and L.M. Levy ABBREVIATIONS: ALS ϭ amyotrophic lateral sclerosis; UMN ϭ upper motor neuron; LMN ϭ lower motor neuron; fALS ϭ familial amyotrophic lateral sclerosis; sALS ϭ sporadic amyotrophic lateral sclerosis myotrophic lateral sclerosis (ALS) reflects a heterogeneous The phenotypic heterogeneity of ALS presents several difficul- Agroup of neurodegenerative disorders unified by loss of mo- ties; the diagnosis of ALS is made on clinical grounds and is fun- tor neurons in the primary motor cortex, brain stem, and spinal damentally uncertain, ranging from “suspected” to “possible,” to cord, resulting in progressive muscle weakness. Typical ALS (rep- “probable” to “definite.” There currently is no stated role for neu- resenting 80% of cases) is a limb-predominant disease character- roimaging to support the diagnosis of ALS; however, diagnosis ized by a combination of upper motor neuron (UMN) and lower requires absence of neuroimaging evidence of other disease pro- motor neuron (LMN) symptoms predominantly affecting the ex- cesses that may explain the observed clinical and electrophysio- tremities. ALS may also present in a bulbar form (20%), with early logical signs.4,5 symptoms involving muscles innervated by the lower brain stem, Absence of a sensitive and specific test for ALS often results in affecting articulation, chewing, and swallowing. While the disease significant delay of diagnosis, which ranges from 13–18 months may chronically persist in such form, it usually progresses to the from onset of the disease or longer in patients who present with 1,2 generalized muscle weakness of typical ALS. isolated LMN signs.5 Furthermore, delayed diagnosis restricts the Patients with ALS are more often male and typically present in inclusion of patients in clinical trials and limits early initiation of late middle age.