Downloads/Oceana-Seafood-Fraud-Report- 21-Feb-2013.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

REPORT on the NORWEGIAN FROZEN FISH FILLET Industryl/ by R
August 1953 Washington 25, D.C. Vol. I 5 , No.8 : REPORT ON THE NORWEGIAN FROZEN FISH FILLET INDUSTRyl/ By R. T. Wh i teleather* FILLETING INDUSTRY IN GENERAL The fisheries that support the groundfish fillet industry in Norway are lo cated in the northern part of the country principally from the Lofot Islands area northward to Hammerfest in Finnmark. These fisheries, particularly those for cod, are generally ranked among the most prolific in the world. In a normal year the Lofot fisheries alone from January through March yield from 80,000 to 120,000 met ric tons of large cod. Notwi t hst anding this, the 1953 spring fishery for cod was disastrous owing to violent storms and almost continuous bad weather for the two- onth period which comprised most of the season. No trawlers operate in t he Lofot cod fishery. The purse seiners, gill netters, ong liner., and hand liners used are dependent to a great extent on reasonably air weather conditions. As a result of the curtailed fishing because of the poor weather during the 1953 ( orwegian Landings of p-r1nc-ipal Species Used in Fillet season, the landings of Industry. 1948- 52 headed and gutted cod asof Species 19521/ 11951 19501194911948 March 30 amounted to only '. Thousands of r.~etric Tons) about 25,000 tons, one of octY (headed and gutted ) . 218 1 249 1961176\ 179 the poorest showings during addock •••••.••• do . ..... 22 18 18 21 25 the past 50 years. Since cean catfish ••• do. ..... 5 6 5 3 1 }'larch is usually considered )cean perch (round) •. -

Honoring the Family Meal Helping Families Be Healthier and Happier with Quick, Easy Seafood Meals
Honoring the Family Meal Helping families be healthier and happier with quick, easy seafood meals OCTOBER IS NATIONAL SEAFOOD MONTH Seafood Nutrition Partnership is here to inspire Americans to enjoy seafood at least twice a week by showing how buying and Fun ways to preparing seafood is simple and delicious! USE THIS TOOLKIT For National Seafood Month in October, we are bringing the This toolkit is designed to inspire and focus back to family. demonstrate how you can implement National Seafood Month this October. Magic happens during family mealtime when children and parents Within the theme, several messaging tracks gather around the table and engage each other in conversation. Family meals eaten at home have been proven to benefit the will be promoted to speak to different health and wellness of children and adolescents, to fight obesity consumers. Please utilize the turnkey and substance abuse, and to make families stronger—creating a resources and messaging, or work with us to positive impact on our communities and our nation as a whole. customize it for your audiences. Join us as we work collaboratively with health partners, ▶ Quick, Easy Weeknight Meals seafood companies, retailers and dietitians from across the Many fish dishes cook in 15 minutes or less country to bring families back to the table. ▶ Fun Ways to Engage Seafood Nutrition Partnership is partnering with the Food Little Seafoodies Marketing Institute Foundation to emphasize the importance of Get kids cooking in the kitchen family meals, expanding National Family Meals Month throughout the year and into a true movement — the Family Meals Movement. -

6 Ounce Whitefish Filets 2 Tablespoons Butter 2 Tablespoons Finely Chopped
3 – 6 ounce whitefish filets 2 tablespoons butter 2 tablespoons finely chopped celery 4 green onions, trimmed and sliced 1 tablespoon flour 1/2 teaspoon Maryland Seafood Seasoning 1/2 teaspoon Lemon & Herb Seasoning 1/2 cup milk 2/3 cup shredded Swiss cheese Preheat oven to 350 degrees. Lightly butter a baking dish. Pat dry the fresh or thawed filets with paper towels and place them in the buttered baking dish. In a small skillet or saucepan melt the butter over low heat. Sauté the celery and green onion for 4 to 5 minutes. Stir in the flour, Maryland Seafood Seasoning, and Lemon & Herb Seasoning. Stir and cook the mixture for about 3 minutes. Gradually blend in the milk while stirring the mixture constantly. After the milk is added and the sauce thickens, stir in the Swiss cheese until it is melted. Pour this sauce over the fish in the baking dish. Bake the fish for about 25 minutes, or until it flakes easily when tested. Makes 3 servings. The familiar species flounder, haddock, cod, basa, and tilapia are examples of whitefish. 2406 Molly Pitcher Highway, Chambersburg, PA 17202 717-263-1214 800-262-1214 www.johnniesinc.com 4 – 6 ounce whitefish fillets 3/4 cup melted butter 2 tablespoons lemon juice 1 cup dry bread crumbs 1/2 cup freshly grated Parmesan cheese Grated Parmesan cheese for sprinkling Preheat oven to 350 degrees. Combine melted butter and lemon juice in a shallow dish. Combine the bread crumbs and Parmesan cheese in a medium size bowl. Dip the fish in the butter mixture, then roll it in the bread crumb-cheese mixture. -

Food Habits of the Southern Channel Catfish (Ictalurus Lacustris Punctatus)
FOOD IIABITS OF TIlE SOUTHERN CHANNEL CATFIStt (ICTALURUS LACUSTRIS PUNCTATUS) IN TItE DES MOINES R,IVER, 'IOWA t I•r:EVE M. BAILEY 2 Muse•,l, of Zoology, U•ffversity of Michigan,, Ann Arbor M•chigan AND H•u•¾ M. H•umso•, J•. Iowa State Co•servcttion(•ommissio,•, Des Moit•cs, Iowa .•BSTRACT The stmnaeh contents of 912 channel catfish (769 containing food) taken iu a short section of the Des Moines River from September, 1940, to October, 1911, are analyzed. The physical and biotic elmraeteristies of the study area are described; a partial list of the fishes present together xvith comments on their importance and relative abundance is included. The ehanuet eatfish is omnivorous, as is revealed by a review of the pertinent literature and by this study. A wide wtriety of organisms is eaten (some 50 families of insects alone are represented--these are listed). Insects and fish serve as staple foods, plant seeds are taken i• season, and various other items are eaten in limited numbers. The principal groups of foods (insects, fish, plants, and miscellaneous) are anMyzed volumetrically, by œrequeney of occurrence, and numerically. In the area studied, catfish grow at a rate of about 4 inches a year during the first 3 years of life (determined by length-frequency analysis). These natural size groups are utilized to establish the relationship between size and food habits. Young fish feed ahnost exclusively on aquatic insect larvae--chiefly midges, blackflies, mayflies, and enddis flies. In fish frmn 4 to 12 inches lo•g insects continue to make up the bulk of the food, but at progressively greater size larger insects (mayflies and caddis flies) are eaten with increasing frequency and dipterans are of less importonce than in the smaller size group; snmll fish and plant seeds become significant items of diet. -

Total Mercury and Copper Concentrations in Lake Trout and Whitefish Fillets from Lake Superior
Total Mercury and Copper Concentrations in Lake Trout and Whitefish Fillets From Lake Superior Activity: 19 -23 By Kory Groetsch Environmental Section Biological Services Division INTRODUCTION Mercury and copper are the two most toxic heavy metals to fish. Furthermore, methyl mercury can accumulate in fish muscle tissue to levels that, consumed on a regular basis, may pose a risk to human health. Both of these metals are released during copper mining and ore processing. Copper mining and processing were a major industry in the Keweenaw Peninsula of Michigan during the mid-1800's. Mining processes which involved heating the ore, released mercury as a bi-product into the air. Furthermore, the unused portion of the ore after the desired minerals were removed (i.e. tailings) were dumped off the shoals of the Keweenaw Peninsula into Lake Superior. These tailing have formed large sholes referred to as stamp sands. Because the extraction of copper from the ore is not a 100% efficient process, it is reasonable to assume that copper, as well as mercury, were in the tailings released into the waters around the Keweenaw Peninsula. The waters around the Keweenaw Peninsula are the location of several lake trout and whitefish spawning reefs. The lake trout and whitefish populations around the Keweenaw Peninsula are sustained by the fish reproduction that occurs on these reefs. These lake trout and whitefish populations have significant cultural and economic importance to the Anishinabe. The purpose of this study was to determine the total mercury and total copper concentrations in the muscle tissue (i.e. fillet) of lake trout and whitefish, in size ranges commonly caught by tribal fishermen around the Keweenaw Penisula. -

Invasive Catfish Management Strategy August 2020
Invasive Catfish Management Strategy August 2020 A team from the Virginia Department of Game and Inland Fisheries uses electrofishing to monitor invasive blue catfish in the James River in 2011. (Photo by Matt Rath/Chesapeake Bay Program) I. Introduction This management strategy portrays the outcomes of an interactive workshop (2020 Invasive Catfish Workshop) held by the Invasive Catfish Workgroup at the Virginia Commonwealth University (VCU) Rice Rivers Center in Charles City, Virginia on January 29-30, 2020. The workshop convened a diverse group of stakeholders to share the current scientific understanding and priority issues associated with invasive catfishes in Chesapeake Bay. The perspectives shared and insights gained from the workshop were used to develop practical, synergistic recommendations that will improve management and mitigate impacts of these species across jurisdictions within the watershed. Blue catfish (Ictalurus furcatus) and flathead catfish (Pylodictis olivaris) are native to the Ohio, Missouri, Mississippi, and Rio Grande river basins, and were introduced into the Virginia tributaries of Chesapeake Bay in the 1960s and 1970s to establish a recreational fishery. These non-native species have since spread, inhabiting nearly all major tributaries of the Bay watershed. Rapid range expansion and population growth, particularly of blue catfish, have led to increasing concerns about impacts on the ecology of the Chesapeake Bay ecosystem. 1 Chesapeake Bay Management Strategy Invasive Catfish Blue and flathead catfishes are long-lived species that can negatively impact native species in Chesapeake Bay through predation and resource competition. Blue catfish are generalist feeders that prey on a wide variety of species that are locally abundant, including those of economic importance and conservation concern, such as blue crabs, alosines, Atlantic menhaden, American eels, and bay anchovy. -

Fresh Product Frozen Product
**PRICING AND AVAILABILITY SUBJECT TO CHANGE** Price U/M Item Item Description/CurbSide Home Delivery Fresh Product 0091 Butter Compound Scampi 9.99/LB 0092 Butter Compound Lemon Dill 9.99/LB 0100 Bluenose Grouper Fillet, Bulk 17.25/LB 0142 Caviar Sturgeon Estate, 1oz. 37.00/LB 0190 Cod Salted, 1 lb. wood box 12.99/LB 0256 Crabmeat JUMBO Lump 12/1 lb. 23.95/LB 0257 Crabmeat Blue Special, 12/1lb. 16.50/LB 0261 Crabmeat Lump Signature Catch 12/1 lb 17.50/LB 0260 Crabmeat Blue Claw , 12/1 lb. 13.50/LB 0069 Grouper Red Fillet Skin Off 18.99/LB 0412 Mahi Mahi S/On Fillet , Bulk 16.99/LB 0451 Ono Fillet Skin On, Bulk 13.95/LB 0655 Fresh Halibut Fillet S/Off 21.99/LB 0705 Rockfish Fillet S/Off, Bulk 5.99/LB 0750 Salmon Atl Fillet 3-4 S/On PBO, Bulk 9.99/LB 0767 Salmon Fillet 3-4 Verlasso S/On, Bulk 12.99/LB 0789 Salmon King N.Z. PBO S/On Fillet, Bulk 18.99/LB 0855 Sea Bass Chilean Fil. S/Off, Bulk 23.99/LB 0870 Scallops Sea U/10 Dry M&B, 1/8#gal 23.95/LB 0904 Sole Petrale Fillet, Bulk 13.99/LB 0905 Swordfish Loin, Bulk 16.99/LB 0932 Tuna Ahi Sashimi Loin , Bulk 20.99/LB 0987 Uzura (Quail Eggs) , 1pk/10pc. 2.99/EA 8605 Beef Wagyu Ribeye Retail, Bulk 29.50/LB Frozen Product 1050 Alligator Tail Meat, 12/1lb. 14.99/LB 1152 Barramundi Fillet S/On Scaled 2/10, Bulk 8.00/LB 1180 Chasen Mongo, 500 G 16.99/EA 1195 Calamari Rings Brd. -

Feeding Catfish in Commercial Ponds
SRAC Publication No. 181 February 2008 VI PR Revision Feeding Catfish in Commercial Ponds Menghe H. Li 1 and Edwin H. Robinson 1 Since feeding is the most impor - to meet the fishes’ total nutritional motes total consumption to avoid tant task in the intensive pond requirements for normal growth waste and higher production cost. production of catfish, the person and development. All catfish feeds Catfish feeds are available as meal responsible for feeding should be are manufactured commercially; (powder), crumbles, and floating an experienced fish culturist who none are prepared on the farm. or slow-sinking pellets. Sinking can tell whether or not the fish are Manufacturers usually produce feeds (prepared in a pellet mill) feeding normally by observing “least-cost” formulations rather are seldom used in catfish produc - them as they come to the surface than “fixed-formula” feeds. In tion. Some producers use sinking to feed. This is generally the only least-cost feed formulation, the medicated feed containing oxyte - time the fish can be seen during formulas vary as ingredient prices tracycline because the antibiotic is grow out. Feeding behavior can be change. However, there are several sensitive to the high heat used in an important clue to the general limitations in the manufacture of the manufacture of floating feeds. health of the fish and the pond catfish feed using least-cost formu - However, there are now floating environment. If the fish are not lations. oxytetracycline-medicated feeds feeding normally, the person who made with “cold-extrusion” tech - is feeding must inform the farm • There is a relatively small num - nology. -
Clean &Unclean Meats
Clean & Unclean Meats God expects all who desire to have a relationship with Him to live holy lives (Exodus 19:6; 1 Peter 1:15). The Bible says following God’s instructions regarding the meat we eat is one aspect of living a holy life (Leviticus 11:44-47). Modern research indicates that there are health benets to eating only the meat of animals approved by God and avoiding those He labels as unclean. Here is a summation of the clean (acceptable to eat) and unclean (not acceptable to eat) animals found in Leviticus 11 and Deuteronomy 14. For further explanation, see the LifeHopeandTruth.com article “Clean and Unclean Animals.” BIRDS CLEAN (Eggs of these birds are also clean) Chicken Prairie chicken Dove Ptarmigan Duck Quail Goose Sage grouse (sagehen) Grouse Sparrow (and all other Guinea fowl songbirds; but not those of Partridge the corvid family) Peafowl (peacock) Swan (the KJV translation of “swan” is a mistranslation) Pheasant Teal Pigeon Turkey BIRDS UNCLEAN Leviticus 11:13-19 (Eggs of these birds are also unclean) All birds of prey Cormorant (raptors) including: Crane Buzzard Crow (and all Condor other corvids) Eagle Cuckoo Ostrich Falcon Egret Parrot Kite Flamingo Pelican Hawk Glede Penguin Osprey Grosbeak Plover Owl Gull Raven Vulture Heron Roadrunner Lapwing Stork Other birds including: Loon Swallow Albatross Magpie Swi Bat Martin Water hen Bittern Ossifrage Woodpecker ANIMALS CLEAN Leviticus 11:3; Deuteronomy 14:4-6 (Milk from these animals is also clean) Addax Hart Antelope Hartebeest Beef (meat of domestic cattle) Hirola chews -

View Take-Out Menu
APPETIZERS Garlic Bread with Marinara .....................................................................................$4.99 Boneless Buffalo or BBQ Wings ...........8 oz Reg $6.99 ..................... 16oz Jumbo $10.99 (Served with blue cheese and celery) Mozzarella Cheese Stix w/ Marinara (6 pcs) ......................................................$4.99 Fried Calamari w/ Marinara Sauce ..................................................................$7.99 Kickin’ Shrimp (Our special fried shrimp tossed in spicy asian sauce) .................dozen $8.99 Steamers (Maine steamed clams. Reg. 1+¼ lb / Lg 2 lb) (when available) ........... market price Authentic Maryland Crabcake Bites........................................................................... $7.99 Basket of Sweet Potato Fries with syrup ................................................................$4.99 Fried Mushroom ......................................................................................................$4.99 Basket of Plantains ..................................................................................$4.99 Basket of Onion Rings .............................................................................................$4.99 SOUPS New England Clam Chowder ......................Cup $3.25......Bowl $4.75........ Quart $9.99 Lobster Bisque w Garlic Bread ..................Cup $4.99......Bowl $8.99..... Quart $19.99 PASTA DISHES SALADS Served With Garlic bread Caesar Salad w. Garlic Bread ............ $4.99 Shrimp Scampi over linguini .......$13.99 -

Southwest Guide: Your Use to Word
BEST CHOICES GOOD ALTERNATIVES AVOID How to Use This Guide Arctic Char (farmed) Clams (US & Canada wild) Bass: Striped (US gillnet, pound net) Bass (US farmed) Cod: Pacific (Canada & US) Basa/Pangasius/Swai Most of our recommendations, Catfish (US) Crab: Southern King (Argentina) Branzino (Mediterranean farmed) including all eco-certifications, Clams (farmed) Lobster: Spiny (US) Cod: Atlantic (gillnet, longline, trawl) aren’t on this guide. Be sure to Cockles Mahi Mahi (Costa Rica, Ecuador, Cod: Pacific (Japan & Russia) Cod: Pacific (AK) Panama & US longlines) Crab (Asia & Russia) check out SeafoodWatch.org Crab: King, Snow & Tanner (AK) Oysters (US wild) Halibut: Atlantic (wild) for the full list. Lobster: Spiny (Belize, Brazil, Lionfish (US) Sablefish/Black Cod (Canada wild) Honduras & Nicaragua) Lobster: Spiny (Mexico) Salmon: Atlantic (BC & ME farmed) Best Choices Mahi Mahi (Peru & Taiwan) Mussels (farmed) Salmon (CA, OR & WA) Octopus Buy first; they’re well managed Oysters (farmed) Shrimp (Canada & US wild, Ecuador, Orange Roughy and caught or farmed responsibly. Rockfish (AK, CA, OR & WA) Honduras & Thailand farmed) Salmon (Canada Atlantic, Chile, Sablefish/Black Cod (AK) Squid (Chile & Peru) Norway & Scotland) Good Alternatives Salmon (New Zealand) Squid: Jumbo (China) Sharks Buy, but be aware there are Scallops (farmed) Swordfish (US, trolls) Shrimp (other imported sources) Seaweed (farmed) Tilapia (Colombia, Honduras Squid (Argentina, China, India, concerns with how they’re Shrimp (US farmed) Indonesia, Mexico & Taiwan) Indonesia, -
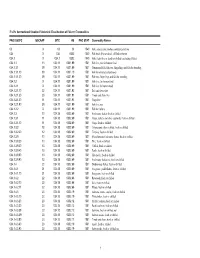
FAO's International Standard Statistical Classification of Fishery Commodities
FAO's International Standard Statistical Classification of Fishery Commodities FAO ISSCFC ISSCAAP SITC HS FAO STAT Commodity Names 03 X 03 03 1540 Fish, crustaceans, molluscs and preparations 034 X 034 0302 1540 Fish fresh (live or dead), chilled or frozen 034.1 X 034.1 0302 1540 Fish, fresh (live or dead) or chilled (excluding fillets) 034.1.1 13 034.11 0301.99 1501 Fish live, not for human food 034.1.1.1 39 034.11 0301.99 1501 Ornamental fish, fish ova, fingerlings and fish for breeding 034.1.1.1.10 39 034.11 0301.10 1501 Fish for ornamental purposes 034.1.1.1.20 39 034.11 0301.99 1501 Fish ova, fingerlings and fish for breeding 034.1.2 X 034.110301.99 1501 Fish live, for human food 034.1.2.1 X 034.110301.99 1501 Fish live for human food 034.1.2.1.10 22 034.11 0301.92 1501 Eels and elvers live 034.1.2.1.20 23 034.11 0301.91 1501 Trouts and chars live 034.1.2.1.30 11 034.11 0301.93 1501 Carps live 034.1.2.1.90 39 034.11 0301.99 1501 Fish live, nei 034.1.2.2 X 034.110301.99 1501 Fish for culture 034.1.3 10 034.18 0302.69 1501 Freshwater fishes, fresh or chilled 034.1.3.1 11 034.18 0302.69 1501 Carps, barbels and other cyprinids, fresh or chilled 034.1.3.1.10 11 034.18 0302.69 1501 Carps, fresh or chilled 034.1.3.2 12 034.18 0302.69 1501 Tilapias and other cichlids, fresh or chilled 034.1.3.2.20 12 034.18 0302.69 1501 Tilapias, fresh or chilled 034.1.3.9 10 034.18 0302.69 1501 Miscellaneous freshwater fishes, fresh or chilled 034.1.3.9.20 13 034.18 0302.69 1501 Pike, fresh or chilled 034.1.3.9.30 13 034.18 0302.69 1501 Catfish, fresh or