Phylum Platyhelminthes (Flatworms) Acoelomates Phylum Nemertina (Ribbon Worms)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intestinal Helminths of the White Sucker, Catostomus Commersoni (Lacepede), in SE Wisconsin
OF WASHINGTON, VOLUME 41, NUMBER 1, JANUARY 1974 81 Intestinal Helminths of the White Sucker, Catostomus commersoni (Lacepede), in SE Wisconsin OMAR M. AMIN Science Division, University of Wisconsin-Parkside, Kenosha 53140 ABSTRACT: Five species of helminths were recovered from the intestine of the white sucker, Catostomus commersoni (Lacepede), in southeastern Wisconsin. Hosts were seined in five sites in both the Root River (Milwaukee and Racine counties; autumn 1971) and the Pike River (Racine and Kenosha counties; autumn 1972). The helminths are Triganodistomum attenuatum Mueller and Van Cleave, 1932 (Trem- atoda: Lissorchiidae), new locality record; Eiacetabulum macrocephalum McCrae, 1962 (Cestoda: Caryophyllaeidae), new state record; Biacetabulum biloculoides Mackiewicz and McCrae, 1965 (Cestoda: Caryophyllaeidae), new state record; Acanthocephahis dims (Van Cleave, 1931) Van Cleave and Townsend, 1936, new host and state record; Dorylaimtis sp. (?) Dujardin, 1845 (Nematoda: Dory- laimidae), a nonparasitie nematode reported for the first time in this fish. Distribution, structural obser- vations, and host-parasite relationships of the above species are discussed. Previous reports of fish parasites in Wis- upon recovery were placed in 70% alcohol. consin were confined to the geographical Hosts were classed in one of three size classes northeast and west (Pearse, 1924a), north according to their total length, 5-9.9, 10-14.9, (Bangham, 1946), northwest (Fischthal, 1947, and 15-37 cm. 1950, 1952), and east (Anthony, 1963). These Trematodes and cestodes were stained in reports dealt only with host records. Literature Semichon's carmine, cleared in xylene, and on the ecology or host-parasite relationship of whole-mounted in Canada balsam. Acantho- fish parasites in Wisconsin is relatively scarce cephalans were fixed in Bouin's fluid, stained (Marshall and Gilbert, 1905; Pearse, 1924b; in Harris' hematoxylin, cleared in beechwood Cross, 1938). -

Number of Living Species in Australia and the World
Numbers of Living Species in Australia and the World 2nd edition Arthur D. Chapman Australian Biodiversity Information Services australia’s nature Toowoomba, Australia there is more still to be discovered… Report for the Australian Biological Resources Study Canberra, Australia September 2009 CONTENTS Foreword 1 Insecta (insects) 23 Plants 43 Viruses 59 Arachnida Magnoliophyta (flowering plants) 43 Protoctista (mainly Introduction 2 (spiders, scorpions, etc) 26 Gymnosperms (Coniferophyta, Protozoa—others included Executive Summary 6 Pycnogonida (sea spiders) 28 Cycadophyta, Gnetophyta under fungi, algae, Myriapoda and Ginkgophyta) 45 Chromista, etc) 60 Detailed discussion by Group 12 (millipedes, centipedes) 29 Ferns and Allies 46 Chordates 13 Acknowledgements 63 Crustacea (crabs, lobsters, etc) 31 Bryophyta Mammalia (mammals) 13 Onychophora (velvet worms) 32 (mosses, liverworts, hornworts) 47 References 66 Aves (birds) 14 Hexapoda (proturans, springtails) 33 Plant Algae (including green Reptilia (reptiles) 15 Mollusca (molluscs, shellfish) 34 algae, red algae, glaucophytes) 49 Amphibia (frogs, etc) 16 Annelida (segmented worms) 35 Fungi 51 Pisces (fishes including Nematoda Fungi (excluding taxa Chondrichthyes and (nematodes, roundworms) 36 treated under Chromista Osteichthyes) 17 and Protoctista) 51 Acanthocephala Agnatha (hagfish, (thorny-headed worms) 37 Lichen-forming fungi 53 lampreys, slime eels) 18 Platyhelminthes (flat worms) 38 Others 54 Cephalochordata (lancelets) 19 Cnidaria (jellyfish, Prokaryota (Bacteria Tunicata or Urochordata sea anenomes, corals) 39 [Monera] of previous report) 54 (sea squirts, doliolids, salps) 20 Porifera (sponges) 40 Cyanophyta (Cyanobacteria) 55 Invertebrates 21 Other Invertebrates 41 Chromista (including some Hemichordata (hemichordates) 21 species previously included Echinodermata (starfish, under either algae or fungi) 56 sea cucumbers, etc) 22 FOREWORD In Australia and around the world, biodiversity is under huge Harnessing core science and knowledge bases, like and growing pressure. -

(Nematoda: Thelazioidea) in the Blue Sucker, Cycleptus Elongatus (Lesueur, 1817), from Illinois
Transactions of the Illinois State Academy of Science received 12/10/01 (2002), Volume 95, #2, pp. 107 - 109 accepted 2/22/02 First Record of Rhabdochona cascadilla Wigdor, 1918 (Nematoda: Thelazioidea) in the Blue Sucker, Cycleptus elongatus (Lesueur, 1817), from Illinois William G. Dyer and William J. Poly Department of Zoology Southern Illinois University Carbondale, Illinois 62901-6501 ABSTRACT Rhabdochona cascadilla was detected in the intestine of Cycleptus elongatus from the Mississippi River in Randolph County, Illinois. This constitutes the first record of this rhabdochonid nematode in the blue sucker and the only internal helminth for this host. INTRODUCTION Nematodes of the genus Rhabdochona Railliet, 1916 (Thelazioidea, Rhabdochonidae) are cosmopolitan in distribution as intestinal parasites of freshwater fishes (Moravec and Coy Otero, 1987; Moravec, 1994). Approximately 96 nominal species have been recognized of which 19 have been reported from North and South America (see Sanchez-Alvarez et al., 1998). Of rhabdochonid nematodes reported from North America, Rhabdochona cascadilla Wigdor, 1918 has been recorded in 13 families, 30 genera, and 53 species of freshwater fishes across Canada and the United States (Hoffman, 1999). Identification of Rhabdochona species is difficult as many of them have been inadequately or erroneously described, and as pointed out by Sanchez-Alvarez et al. (1998), a detailed taxonomic revision of all putative species in this group warrants initiation. MATERIALS AND METHODS A single adult female blue sucker (557 mm standard length) was collected incidentally by electrofishing on 24 October 1996 from the Mississippi River just below the mouth of the Kaskaskia River, Randolph County, Illinois and was transported alive to the laboratory. -

Digenean Metacercaria (Trematoda, Digenea, Lepocreadiidae) Parasitizing “Coelenterates” (Cnidaria, Scyphozoa and Ctenophora) from Southeastern Brazil
BRAZILIAN JOURNAL OF OCEANOGRAPHY, 53(1/2):39-45, 2005 DIGENEAN METACERCARIA (TREMATODA, DIGENEA, LEPOCREADIIDAE) PARASITIZING “COELENTERATES” (CNIDARIA, SCYPHOZOA AND CTENOPHORA) FROM SOUTHEASTERN BRAZIL André Carrara Morandini1; Sergio Roberto Martorelli2; Antonio Carlos Marques1 & Fábio Lang da Silveira1 1Instituto de Biociências da Universidade de São Paulo Departamento de Zoologia (Caixa Postal 11461, 05422-970, São Paulo, SP, Brazil) 2Centro de Estudios Parasitologicos y Vectores (CEPAVE) (2 Nro. 584 (1900) La Plata, Argentina) e-mails: [email protected], [email protected], [email protected], [email protected] A B S T R A C T Metacercaria specimens of the genus Opechona (Trematoda: Digenea: Lepocreadiidae) are described parasitizing “coelenterates” (scyphomedusae and ctenophores) from Southeastern Brazil (São Paulo state). The worms are compared to other Opechona species occurring on the Brazilian coast, but no association has been made because only adult forms of these species have been described. Suppositions as to the possible transference of the parasites are made. R E S U M O Exemplares de metacercárias do gênero Opechona (Trematoda: Digenea: Lepocreadiidae) são descritos parasitando “celenterados” (cifomedusas e ctenóforos) no sudeste do Brasil (estado de São Paulo). Os vermes foram comparados a outras espécies de Opechona ocorrentes no litoral brasileiro, porém nenhuma associação foi realizada devido às demais espécies terem sido descritas a partir de exemplares adultos. São apresentadas suposições sobre as possíveis formas -

Shortnose Sucker (Chasmistes Brevirostris) 5-Year Review Summary and Evaluation
Shortnose Sucker (Chasmistes brevirostris) 5-Year Review Summary and Evaluation U.S. Fish and Wildlife Service Klamath Falls Fish and Wildlife Office Klamath Falls, Oregon July 2007 5-YEAR REVIEW Shortnose Sucker (Chasmistes brevirostris) TABLE OF CONTENTS 1.0 GENERAL INFORMATION.......................................................................................... 3 1.1. Reviewers............................................................................................................................ 3 1.2. Methodology used to complete the review....................................................................... 3 1.3. Background ........................................................................................................................ 3 2.0 REVIEW ANALYSIS....................................................................................................... 4 2.1. Application of the 1996 Distinct Populations Segment (DPS) policy ............................ 4 2.2. Biology and Habitat ........................................................................................................... 5 2.3. Recovery Criteria............................................................................................................. 13 2.4. Five-Factor Analysis ........................................................................................................ 16 2.5. Synthesis............................................................................................................................ 30 3.0 RESULTS ....................................................................................................................... -

Symbiosis (Symbiotic Relationship)
Symbiosis (Symbiotic Relationship) 1 In the wonderful world of nature, some animals love forming partnerships with other animal species, with plants, and with microorganisms. We have a special name for such interesting arrangements. We call it "symbiosis" that literally means "living together". 2 Do both species involved in a symbiotic relationship benefit from their partnership? Well, the question itself is open for debate. While some scientists restrict the meaning of symbiosis to a "win-win" situation for both participants, others disagree. Using a broader definition, we are going to explore the three types of symbiotic partnerships. 3 When two species engage in a mutually beneficial symbiotic relationship, they are in the so- called "mutualism" type of symbiosis. To understand mutualism better, let's examine the interaction between clown fish and an anemone. While most fish stay away from an anemone for fear of touching its poisonous tentacles, clown fish have a special coat on their skin that protects them from getting stung. (This trick does not work for all anemones though. Clown fish can only have symbiotic relationships with 10 of the 1,000 different anemone species in the world.) Swimming carefree and unharmed among their host's deadly tentacles, clown fish know very well that their predators do not dare to come near them. Plus, clown fish get to pick up and eat the leftover bits discarded by their landlord. What does the anemone get in return for offering clown fish a safe haven? Well, first and foremost, it kills and feeds on fish that are eyeing its tenant! Aside from that, clown fish pay their rent by cleaning up food scraps and dead anemone tentacles. -

Helminth Parasites (Trematoda, Cestoda, Nematoda, Acanthocephala) of Herpetofauna from Southeastern Oklahoma: New Host and Geographic Records
125 Helminth Parasites (Trematoda, Cestoda, Nematoda, Acanthocephala) of Herpetofauna from Southeastern Oklahoma: New Host and Geographic Records Chris T. McAllister Science and Mathematics Division, Eastern Oklahoma State College, Idabel, OK 74745 Charles R. Bursey Department of Biology, Pennsylvania State University-Shenango, Sharon, PA 16146 Matthew B. Connior Life Sciences, Northwest Arkansas Community College, Bentonville, AR 72712 Abstract: Between May 2013 and September 2015, two amphibian and eight reptilian species/ subspecies were collected from Atoka (n = 1) and McCurtain (n = 31) counties, Oklahoma, and examined for helminth parasites. Twelve helminths, including a monogenean, six digeneans, a cestode, three nematodes and two acanthocephalans was found to be infecting these hosts. We document nine new host and three new distributional records for these helminths. Although we provide new records, additional surveys are needed for some of the 257 species of amphibians and reptiles of the state, particularly those in the western and panhandle regions who remain to be examined for helminths. ©2015 Oklahoma Academy of Science Introduction Methods In the last two decades, several papers from Between May 2013 and September 2015, our laboratories have appeared in the literature 11 Sequoyah slimy salamander (Plethodon that has helped increase our knowledge of sequoyah), nine Blanchard’s cricket frog the helminth parasites of Oklahoma’s diverse (Acris blanchardii), two eastern cooter herpetofauna (McAllister and Bursey 2004, (Pseudemys concinna concinna), two common 2007, 2012; McAllister et al. 1995, 2002, snapping turtle (Chelydra serpentina), two 2005, 2010, 2011, 2013, 2014a, b, c; Bonett Mississippi mud turtle (Kinosternon subrubrum et al. 2011). However, there still remains a hippocrepis), two western cottonmouth lack of information on helminths of some of (Agkistrodon piscivorus leucostoma), one the 257 species of amphibians and reptiles southern black racer (Coluber constrictor of the state (Sievert and Sievert 2011). -

Identity of Diphyllobothrium Spp. (Cestoda: Diphyllobothriidae) from Sea Lions and People Along the Pacific Coast of South America
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Laboratory of Parasitology Parasitology, Harold W. Manter Laboratory of 4-2010 Identity of Diphyllobothrium spp. (Cestoda: Diphyllobothriidae) from Sea Lions and People along the Pacific Coast of South America Robert L. Rausch University of Washington, [email protected] Ann M. Adams United States Food and Drug Administration Leo Margolis Fisheries Research Board of Canada Follow this and additional works at: https://digitalcommons.unl.edu/parasitologyfacpubs Part of the Parasitology Commons Rausch, Robert L.; Adams, Ann M.; and Margolis, Leo, "Identity of Diphyllobothrium spp. (Cestoda: Diphyllobothriidae) from Sea Lions and People along the Pacific Coast of South America" (2010). Faculty Publications from the Harold W. Manter Laboratory of Parasitology. 498. https://digitalcommons.unl.edu/parasitologyfacpubs/498 This Article is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications from the Harold W. Manter Laboratory of Parasitology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. J. Parasitol., 96(2), 2010, pp. 359–365 F American Society of Parasitologists 2010 IDENTITY OF DIPHYLLOBOTHRIUM SPP. (CESTODA: DIPHYLLOBOTHRIIDAE) FROM SEA LIONS AND PEOPLE ALONG THE PACIFIC COAST OF SOUTH AMERICA Robert L. Rausch, Ann M. Adams*, and Leo MargolisÀ Department of Comparative Medicine and Department of Pathobiology, Box 357190, University of Washington, Seattle, Washington 98195-7190. e-mail: [email protected] ABSTRACT: Host specificity evidently is not expressed by various species of Diphyllobothrium that occur typically in marine mammals, and people become infected occasionally when dietary customs favor ingestion of plerocercoids. -

Animal Phylum Poster Porifera
Phylum PORIFERA CNIDARIA PLATYHELMINTHES ANNELIDA MOLLUSCA ECHINODERMATA ARTHROPODA CHORDATA Hexactinellida -- glass (siliceous) Anthozoa -- corals and sea Turbellaria -- free-living or symbiotic Polychaetes -- segmented Gastopods -- snails and slugs Asteroidea -- starfish Trilobitomorpha -- tribolites (extinct) Urochordata -- tunicates Groups sponges anemones flatworms (Dugusia) bristleworms Bivalves -- clams, scallops, mussels Echinoidea -- sea urchins, sand Chelicerata Cephalochordata -- lancelets (organisms studied in detail in Demospongia -- spongin or Hydrazoa -- hydras, some corals Trematoda -- flukes (parasitic) Oligochaetes -- earthworms (Lumbricus) Cephalopods -- squid, octopus, dollars Arachnida -- spiders, scorpions Mixini -- hagfish siliceous sponges Xiphosura -- horseshoe crabs Bio1AL are underlined) Cubozoa -- box jellyfish, sea wasps Cestoda -- tapeworms (parasitic) Hirudinea -- leeches nautilus Holothuroidea -- sea cucumbers Petromyzontida -- lamprey Mandibulata Calcarea -- calcareous sponges Scyphozoa -- jellyfish, sea nettles Monogenea -- parasitic flatworms Polyplacophora -- chitons Ophiuroidea -- brittle stars Chondrichtyes -- sharks, skates Crustacea -- crustaceans (shrimp, crayfish Scleropongiae -- coralline or Crinoidea -- sea lily, feather stars Actinipterygia -- ray-finned fish tropical reef sponges Hexapoda -- insects (cockroach, fruit fly) Sarcopterygia -- lobed-finned fish Myriapoda Amphibia (frog, newt) Chilopoda -- centipedes Diplopoda -- millipedes Reptilia (snake, turtle) Aves (chicken, hummingbird) Mammalia -

Invertebrates Invertebrates: • Are Animals Without Backbones • Represent 95% of the Animal Kingdom Animal Diversity Morphological Vs
Invertebrates Invertebrates: • Are animals without backbones • Represent 95% of the animal kingdom Animal Diversity Morphological vs. Molecular Character Phylogeny? A tree is a hypothesis supported or not supported by evidence. Groupings change as new evidence become available. Sponges - Porifera Natural Bath Sponges – over-collected, now uncommon Sponges • Perhaps oldest animal phylum (Ctenphora possibly older) • may represent several old phyla, some now extinct ----------------Ctenophora? Sponges - Porifera • Mostly marine • Sessile animals • Lack true tissues; • Have only a few cell types, cells kind of independent • Most have no symmetry • Body resembles a sac perforated with holes, system of canals. • Strengthened by fibers of spongin, spicules Sponges have a variety of shapes Sponges Pores Choanocyte Amoebocyte (feeding cell) Skeletal Water fiber flow Central cavity Flagella Choanocyte in contact with an amoebocyte Sponges - Porifera • Sessile filter feeder • No mouth • Sac-like body, perforated by pores. • Interior lined by flagellated cells (choanocytes). Flagellated collar cells generate a current, draw water through the walls of the sponge where food is collected. • Amoeboid cells move around in the mesophyll and distribute food. Sponges - Porifera Grantia x.s. Sponge Reproduction Asexual reproduction • Fragmentation or by budding. • Sponges are capable of regeneration, growth of a whole from a small part. Sexual reproduction • Hermaphrodites, produce both eggs and sperm • Eggs and sperm released into the central cavity • Produces -

Myzopodidae: Chiroptera) from Western Madagascar
ARTICLE IN PRESS www.elsevier.de/mambio Original investigation The description of a new species of Myzopoda (Myzopodidae: Chiroptera) from western Madagascar By S.M. Goodman, F. Rakotondraparany and A. Kofoky Field Museum of Natural History, Chicago, USA and WWF, Antananarivo, De´partement de Biologie Animale, Universite´ d’Antananarivo, Antananarivo, Madagasikara Voakajy, Antananarivo, Madagascar Receipt of Ms. 6.2.2006 Acceptance of Ms. 2.8.2006 Abstract A new species of Myzopoda (Myzopodidae), an endemic family to Madagascar that was previously considered to be monospecific, is described. This new species, M. schliemanni, occurs in the dry western forests of the island and is notably different in pelage coloration, external measurements and cranial characters from M. aurita, the previously described species, from the humid eastern forests. Aspects of the biogeography of Myzopoda and its apparent close association with the plant Ravenala madagascariensis (Family Strelitziaceae) are discussed in light of possible speciation mechanisms that gave rise to eastern and western species. r 2006 Deutsche Gesellschaft fu¨r Sa¨ugetierkunde. Published by Elsevier GmbH. All rights reserved. Key words: Myzopoda, Madagascar, new species, biogeography Introduction Recent research on the mammal fauna of speciation molecular studies have been very Madagascar has and continues to reveal informative to resolve questions of species remarkable discoveries. A considerable num- limits (e.g., Olson et al. 2004; Yoder et al. ber of new small mammal and primate 2005). The bat fauna of the island is no species have been described in recent years exception – until a decade ago these animals (Goodman et al. 2003), and numerous remained largely under studied and ongoing other mammals, known to taxonomists, surveys and taxonomic work have revealed await formal description. -

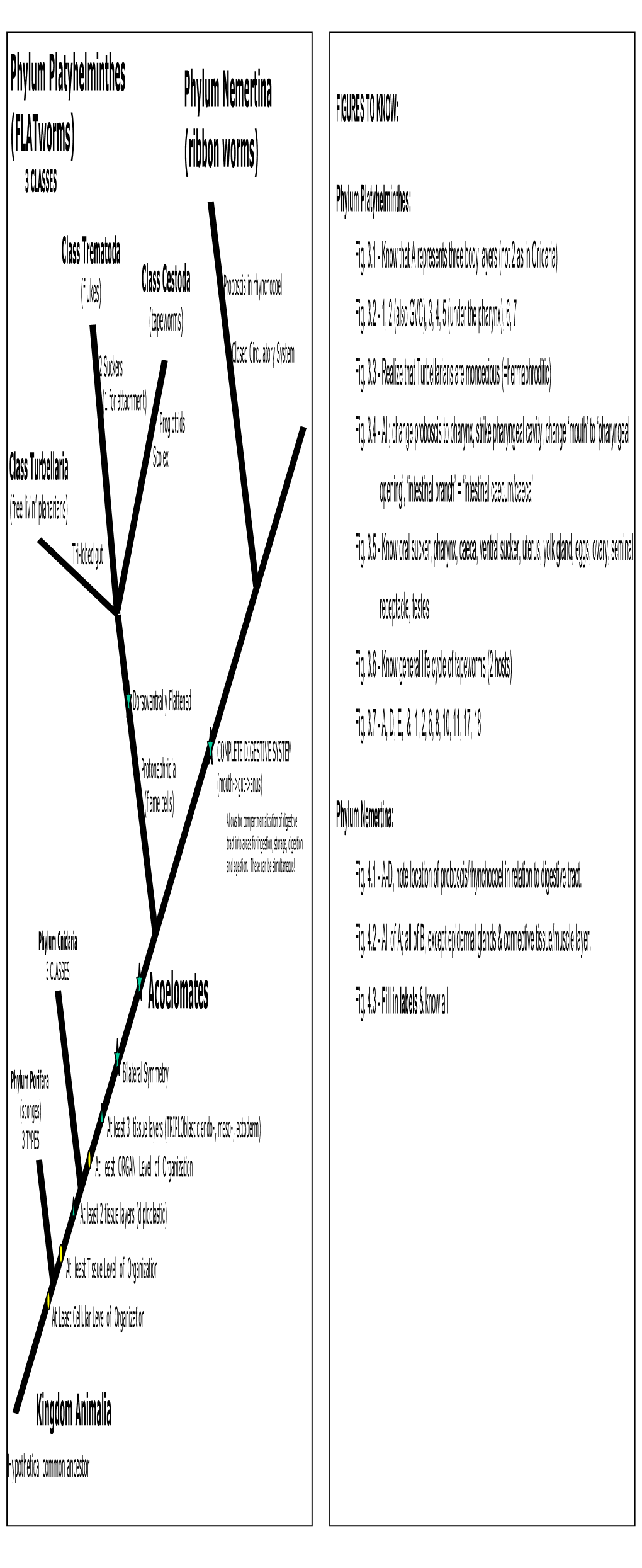
R E S E a R C H / M a N a G E M E N T Aquatic and Terrestrial Flatworm (Platyhelminthes, Turbellaria) and Ribbon Worm (Nemertea)
RESEARCH/MANAGEMENT FINDINGSFINDINGS “Put a piece of raw meat into a small stream or spring and after a few hours you may find it covered with hundreds of black worms... When not attracted into the open by food, they live inconspicuously under stones and on vegetation.” – BUCHSBAUM, et al. 1987 Aquatic and Terrestrial Flatworm (Platyhelminthes, Turbellaria) and Ribbon Worm (Nemertea) Records from Wisconsin Dreux J. Watermolen D WATERMOLEN Bureau of Integrated Science Services INTRODUCTION The phylum Platyhelminthes encompasses three distinct Nemerteans resemble turbellarians and possess many groups of flatworms: the entirely parasitic tapeworms flatworm features1. About 900 (mostly marine) species (Cestoidea) and flukes (Trematoda) and the free-living and comprise this phylum, which is represented in North commensal turbellarians (Turbellaria). Aquatic turbellari- American freshwaters by three species of benthic, preda- ans occur commonly in freshwater habitats, often in tory worms measuring 10-40 mm in length (Kolasa 2001). exceedingly large numbers and rather high densities. Their These ribbon worms occur in both lakes and streams. ecology and systematics, however, have been less studied Although flatworms show up commonly in invertebrate than those of many other common aquatic invertebrates samples, few biologists have studied the Wisconsin fauna. (Kolasa 2001). Terrestrial turbellarians inhabit soil and Published records for turbellarians and ribbon worms in leaf litter and can be found resting under stones, logs, and the state remain limited, with most being recorded under refuse. Like their freshwater relatives, terrestrial species generic rubric such as “flatworms,” “planarians,” or “other suffer from a lack of scientific attention. worms.” Surprisingly few Wisconsin specimens can be Most texts divide turbellarians into microturbellarians found in museum collections and a specialist has yet to (those generally < 1 mm in length) and macroturbellari- examine those that are available.