University of Birmingham Quantifying the Completeness of the Bat Fossil
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microchiroptera: Mystacinidae) from Australia, with a Revised Diagnosis of the Genus
New Miocene Icarops material (Microchiroptera: Mystacinidae) from Australia, with a revised diagnosis of the genus SUZANNE HAND, MICHAEL ARCHER & HENK GODTHELP HAND, S.l., ARCHER, M. & GODTHELP, H., 2001:12:20. New Miocene lcarops material (Microchiroptera: Mystacinidae) from Australia, with a revised diagnosis of the genus. Memoirs of the Association of Australasian Palaeontologists 25,139-146. ISSN 0810-8889 New fossil material referable to Icarops paradox Hand et al., 1998 is described from the early Miocene Judith's Horizontalis Site in the Riversleigh World Heritage Property of northwestern Queensland. Fused dentaries contain the partial lower dentition of I. paradox. The diagnosis of the genus Icarops is revised. The new material confirms the identity of Icarops species as mystacinids and enablesre-examination of interrelationships between extinct and extant members of this Gondwanan bat family. S.J: Hand, M. Archer* & H. Godthelp, School of Biological Science, University of New South Wales,New South Wales, 2052; * also Australian Museum, 6-8 College St, Sydney, New South Wales,2000. Received ]4 December 2000 Keywords: Mystacinidae, Icarops, Mystacina, bat, lower dentition, Miocene, Riversleigh THE FIRST pre-Pleistocene record for the QMF refers to specimens held in the fossil Mystacinidae and first record of this bat family collections of the QueenslandMuseum, Brisbane. from outside New Zealand were reported by Hand et al. ( 1998) from Miocene sedimentsin Australia. SYSTEMAllC PALAEONTOLOGY Three species of the new mystacinid genus Icarops were described: Icarops breviceps from OrderCIllROPTERAB1wnenbach, 1779 the middle Miocene Bullock Creek deposit of the SuborderMICROCIllROPTERA Dobson, 1875 Northern Territory; I. aenae from the early SuperfamilyNocmIoNoIDEA Van Va1en, Miocene Wayne's Wok deposit, D Site Plateau, 1979 Riversleigh, northwestern Queensland; and I. -

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -
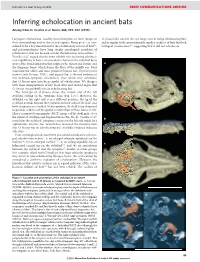
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -

Origin and Beyond
EVOLUTION ORIGIN ANDBEYOND Gould, who alerted him to the fact the Galapagos finches ORIGIN AND BEYOND were distinct but closely related species. Darwin investigated ALFRED RUSSEL WALLACE (1823–1913) the breeding and artificial selection of domesticated animals, and learned about species, time, and the fossil record from despite the inspiration and wealth of data he had gathered during his years aboard the Alfred Russel Wallace was a school teacher and naturalist who gave up teaching the anatomist Richard Owen, who had worked on many of to earn his living as a professional collector of exotic plants and animals from beagle, darwin took many years to formulate his theory and ready it for publication – Darwin’s vertebrate specimens and, in 1842, had “invented” the tropics. He collected extensively in South America, and from 1854 in the so long, in fact, that he was almost beaten to publication. nevertheless, when it dinosaurs as a separate category of reptiles. islands of the Malay archipelago. From these experiences, Wallace realized By 1842, Darwin’s evolutionary ideas were sufficiently emerged, darwin’s work had a profound effect. that species exist in variant advanced for him to produce a 35-page sketch and, by forms and that changes in 1844, a 250-page synthesis, a copy of which he sent in 1847 the environment could lead During a long life, Charles After his five-year round the world voyage, Darwin arrived Darwin saw himself largely as a geologist, and published to the botanist, Joseph Dalton Hooker. This trusted friend to the loss of any ill-adapted Darwin wrote numerous back at the family home in Shrewsbury on 5 October 1836. -

Attachment J Assessment of Existing Paleontologic Data Along with Field Survey Results for the Jonah Field
Attachment J Assessment of Existing Paleontologic Data Along with Field Survey Results for the Jonah Field June 12, 2007 ABSTRACT This is compilation of a technical analysis of existing paleontological data and a limited, selective paleontological field survey of the geologic bedrock formations that will be impacted on Federal lands by construction associated with energy development in the Jonah Field, Sublette County, Wyoming. The field survey was done on approximately 20% of the field, primarily where good bedrock was exposed or where there were existing, debris piles from recent construction. Some potentially rich areas were inaccessible due to biological restrictions. Heavily vegetated areas were not examined. All locality data are compiled in the separate confidential appendix D. Uinta Paleontological Associates Inc. was contracted to do this work through EnCana Oil & Gas Inc. In addition BP and Ultra Resources are partners in this project as they also have holdings in the Jonah Field. For this project, we reviewed a variety of geologic maps for the area (approximately 47 sections); none of maps have a scale better than 1:100,000. The Wyoming 1:500,000 geology map (Love and Christiansen, 1985) reveals two Eocene geologic formations with four members mapped within or near the Jonah Field (Wasatch – Alkali Creek and Main Body; Green River – Laney and Wilkins Peak members). In addition, Winterfeld’s 1997 paleontology report for the proposed Jonah Field II Project was reviewed carefully. After considerable review of the literature and museum data, it became obvious that the portion of the mapped Alkali Creek Member in the Jonah Field is probably misinterpreted. -

Rapid and Early Post-Flood Mammalian Diversification Videncede in the Green River Formation
The Proceedings of the International Conference on Creationism Volume 6 Print Reference: Pages 449-457 Article 36 2008 Rapid and Early Post-Flood Mammalian Diversification videncedE in the Green River Formation John H. Whitmore Cedarville University Kurt P. Wise Southern Baptist Theological Seminary Follow this and additional works at: https://digitalcommons.cedarville.edu/icc_proceedings DigitalCommons@Cedarville provides a publication platform for fully open access journals, which means that all articles are available on the Internet to all users immediately upon publication. However, the opinions and sentiments expressed by the authors of articles published in our journals do not necessarily indicate the endorsement or reflect the views of DigitalCommons@Cedarville, the Centennial Library, or Cedarville University and its employees. The authors are solely responsible for the content of their work. Please address questions to [email protected]. Browse the contents of this volume of The Proceedings of the International Conference on Creationism. Recommended Citation Whitmore, John H. and Wise, Kurt P. (2008) "Rapid and Early Post-Flood Mammalian Diversification Evidenced in the Green River Formation," The Proceedings of the International Conference on Creationism: Vol. 6 , Article 36. Available at: https://digitalcommons.cedarville.edu/icc_proceedings/vol6/iss1/36 In A. A. Snelling (Ed.) (2008). Proceedings of the Sixth International Conference on Creationism (pp. 449–457). Pittsburgh, PA: Creation Science Fellowship and Dallas, TX: Institute for Creation Research. Rapid and Early Post-Flood Mammalian Diversification Evidenced in the Green River Formation John H. Whitmore, Ph.D., Cedarville University, 251 N. Main Street, Cedarville, OH 45314 Kurt P. Wise, Ph.D., Southern Baptist Theological Seminary, 2825 Lexington Road. -

Bats NW Spring 08
Become a Bats Northwest Member Become a Bats Northwest Member. Join us in the adventure to learn more about our bat neighbors! BatsBats News Membership Options: $35 $50 $75 $100 Other $_________________ Name:_________________________________________________________________________________ Address:______________________________________________________________________________ Bats Northwest Mailing Address: NorthwestNorthwest P.O. Box 3026 _______________________________________________________________________________________ BNW IS A NON-PROFIT, ALL VOLUNTEER CONSERVATION ORGANIZATION SPRING 2008 Lynnwood, WA 98046 Phone:_________________________________________________________________________________ Bats and Humans: provide as much as 40% of the lift force that 206.256.0406 Email:_________________________________________________________________________________ helped the bats stay aloft. A Helping Hand Bats Northwest web site: Bats go a step further by using their embedded www.batsnorthwest.org by Kathleen Bander thumbs and fingers as flaps like those on an airplane wing, to alter the curve of the wing BATS NW T-SHIRTS What amazing adaptation do bats and insects and create the lift force needed to hover. have in common? No, it isn’t just that insects You’ll look great in our Bats Northwest Long-Sleeved T-Shirt! It also makes a are food for bats. Nor is it that flight is the main Scientists found that the reason bats are able wonderful gift. mode of transportation for both. to produce LEVs is because they can actively Heavyweight cotton, natural off-white, with a brightly colored bat graphic. change the curvature of their wings using their The commonality is the ability, only lately elongaged fingers and muscle fibers unlike I WOULD LIKE TO ORDER ____ (QUANTITY) BATS NORTHWEST LONG-SLEEVED T-SHIRT(S) AT $22.00 EACH FOR witnessed by scientists, of both bats and insects which rely on extreme speed. -

Integrated Fossil and Molecular Data Reconstruct Bat Echolocation
Integrated fossil and molecular data reconstruct bat echolocation Mark S. Springer†‡, Emma C. Teeling†§, Ole Madsen¶, Michael J. Stanhope§ʈ, and Wilfried W. de Jong¶** †Department of Biology, University of California, Riverside, CA 92521; §Queen’s University of Belfast, Biology and Biochemistry, 97 Lisburn Road, Belfast BT9 7BL, United Kingdom; ¶Department of Biochemistry, University of Nijmegen, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands; ʈBioinformatics, SmithKline Beecham Pharmaceuticals, 1250 South Collegeville Road, UP1345, Collegeville, PA 19426; and **Institute for Biodiversity and Ecosystem Dynamics, 1090 GT Amsterdam, The Netherlands Edited by David Pilbeam, Harvard University, Cambridge, MA, and approved March 26, 2001 (received for review November 21, 2000) Molecular and morphological data have important roles in illumi- tulates a sister-group relationship between flying lemurs and bats nating evolutionary history. DNA data often yield well resolved (6). Although some authors have questioned bat monophyly phylogenies for living taxa, but are generally unattainable for based on evidence from the penis and nervous system (7, 8), the fossils. A distinct advantage of morphology is that some types of bulk of morphological data supports bat monophyly (9). Among morphological data may be collected for extinct and extant taxa. living Chiroptera, morphological data provide strong support for Fossils provide a unique window on evolutionary history and may the reciprocal monophyly of megachiropterans (Old World fruit preserve combinations -

Biogeography and Phylogeny of the Eutheria
FAUNA of AUSTRALIA 36. BIOGEOGRAPHY AND PHYLOGENY OF EUTHERIA G.M. MCKAY, J.H. CALABY & L.S. HALL 1 36. BIOGEOGRAPHY AND PHYLOGENY OF EUTHERIA 2 36. BIOGEOGRAPHY AND PHYLOGENY OF EUTHERIA INTRODUCTION In considering the biogeography and phylogeny of the eutherian mammals in Australia, a distinction must be made between those groups which invaded the continent prior to the 18th Century and those that have been deliberately or inadvertently introduced since then. For the latter group, the worldwide distribution and fossil history is essentially irrelevant except that many species have a long association with humans as either domesticated animals or commensals. In this chapter, we will address the more general questions of the origins and affinities of the native Australian eutherians. For details of the biogeography, fossil history and affinities of the introduced mammals, the reader should refer to the family accounts (Chapters 44–46, 54, 55, 58–62). Any examination of the phylogeny of a group must consider two main lines of evidence: the fossil record and the study of living forms. Morphological studies can be made on both living and fossil forms and such studies, particularly of skeletal and dental characters, have traditionally formed the basis for phylogenetic reconstruction in the Mammalia. In recent years there has been an increase in biochemical and molecular studies on living species which, with a very few exceptions, cannot be compared to extinct species. To date, only a relatively few species of living mammals have been studied adequately using molecular techniques, but attempts are being made to reconcile the biochemical data with the traditional morphological studies (Shoshani 1986; McKenna 1987). -

Geology* Paleontology, and Correlation of Eocene Volcaniclastic Rocks, Southeast Absaroka Range, Hot Springs County, Wyoming
Geology* Paleontology, and Correlation of Eocene Volcaniclastic Rocks, Southeast Absaroka Range, Hot Springs County, Wyoming GEOLOGICAL SURVEY PROFESSIONAL PAPER 1201-A Geology, Paleontology, and Correlation of Eocene Volcaniclastic Rocks, Southeast Absaroka Range, Hot Springs County, Wyoming By T. M. BOWN GEOLOGY OF THE ABSAROKA RANGE, NORTHWEST WYOMING GEOLOGICAL SURVEY PROFESSIONAL PAPER 1201-A Relations of Eocene volcaniclastic rocks and Eocene fossils date the sedimentary and structural evolution of the southeast Absaroka Range, clarify correlations with adjacent areas, and aid in timing large-scale events of gravity faulting UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1982 UNITED STATES DEPARTMENT OF THE INTERIOR JAMES G. WATT, Secretary GEOLOGICAL SURVEY Dallas L. Peck, Director Library of Congress Cataloging in Publication Data Bown, Thomas M. Geology, paleontology, and correlation of Eocene volcaniclastic rocks, southeast Absaroka Range, Hot Springs County, Wyoming. (Geological Survey Professional Paper 1201-A) Bibliography: p.71 Supt.ofDocs.no.: II9.I6:1201-A 1. Geology, Stratigraphic Eocene. 2. Volcanic ash, tuff, etc. Wyoming Hot Springs Co. 3. Mammals, Fossil. 4. Stratigraphic correlation Wyoming Hot Springs Co. 5. Geology Wyoming Hot Springs Co. 1. Title. II. Series: United States. Geological Survey. Professional Paper 1201-A. QE692.2.B68 55I.7'84'0978743 80-607125 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 CONTENTS Page Abstract ------------------------------------------------- -
The Lung-Air Sac System of Birds
The Lung-Air Sac System of Birds Development, Structure, and Function Bearbeitet von John N. Maina 1. Auflage 2005. Buch. xvi, 210 S. Hardcover ISBN 978 3 540 25595 6 Format (B x L): 15,5 x 23,5 cm Gewicht: 550 g Weitere Fachgebiete > Chemie, Biowissenschaften, Agrarwissenschaften > Wirbeltiere (Vertebrata) > Vögel (Ornithologie) Zu Inhaltsverzeichnis schnell und portofrei erhältlich bei Die Online-Fachbuchhandlung beck-shop.de ist spezialisiert auf Fachbücher, insbesondere Recht, Steuern und Wirtschaft. Im Sortiment finden Sie alle Medien (Bücher, Zeitschriften, CDs, eBooks, etc.) aller Verlage. Ergänzt wird das Programm durch Services wie Neuerscheinungsdienst oder Zusammenstellungen von Büchern zu Sonderpreisen. Der Shop führt mehr als 8 Millionen Produkte. 1 Flight Muscle powered flight requires a high meta- bolic rate and a very efficient respiratory sys- tem. Constable (1990) 1.1 Energetic Cost of Flight Defined as capacity to produce lift, accelerate, and maneuver at various speeds, powered flight is an elite form of locomotion. It has compelled incom- parable structural specializations and refinements and striking functional integration of practically all the organs and organ systems, especially the gas- trointestinal, cardiovascular, respiratory, muscular, and nervous systems. Exerting substantial metabolic,mechanical,and aerodynamic demands,flight adaptively evolved in response to particular selective pressures in order to meet specific survival needs. To morphologists and physiologists, in many ways volant animals offer exceptional -
An Ailuravine Rodent from the Lower Eocene Cambay Formation at Vastan, Western India, and Its Palaeobiogeographic Implications
An ailuravine rodent from the lower Eocene Cambay Formation at Vastan, western India, and its palaeobiogeographic implications RAJENDRA S. RANA, KISHOR KUMAR, GILLES ESCARGUEL, ASHOK SAHNI, KENNETH D. ROSE, THIERRY SMITH, HUKAM SINGH, and LACHHAM SINGH Rana, R.S., Kumar, K., Escarguel, G., Sahni, A., Rose, K.D., Smith, T., Singh, H., and Singh, L. 2008. An ailuravine ro− dent from the lower Eocene Cambay Formation at Vastan, western India, and its palaeobiogeographic implications. Acta Palaeontologica Polonica 53 (1): 1–14. A new ailuravine rodent, Meldimys musak sp. nov. (Mammalia: Rodentia, Ischyromyidae), is recorded from the lower Eocene lignites of western India. It is the oldest record of Rodentia from India. M. musak is more derived than the earliest Eocene ailuravine Euromys cardosoi from Portugal and more generalized than late early Eocene E. inexpectatus and Ailuravus michauxi from France. Its dental morphology closely corresponds to the middle early Eocene species M. louisi, which lived about 52 Ma in Western Europe. Meldimys was previously known only from Europe, and ailuravines were previously reported only from Europe and North America. Its occurrence in India allows the first direct correlation be− tween the early Eocene land mammal horizons of Europe and India, and raises the possibility of a terrestrial faunal ex− change between India and Eurasia close to the Palaeocene–Eocene transition. Key words: Mammalia, Ailuravinae, Rodentia, palaeobiogeography, Eocene, India. Rajendra S. Rana [[email protected]] and Lachham Singh [[email protected]], Department of Geology, HNB Garhwal University, Srinagar 246 175 UA, India; Kishor Kumar [[email protected]], Wadia Institute of Himalayan Geology, 33 General Mahadeo Singh Road, Dehradun 248 001 UA, India (corresponding author); Gilles Escarguel [gilles.escarguel@univ−lyon1.fr], UMR CNRS 5125 “Paléoenvironnements & Paléobiosphère”, France; Université Lyon 1, Campus de la Doua, Bât.