Streptococcus Gordonii: Pathogenesis and Host Response to Its Cell Wall Components
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
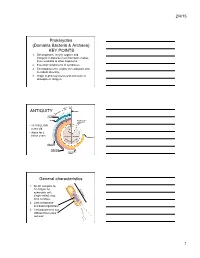
Prokaryotes (Domains Bacteria & Archaea)
2/4/15 Prokaryotes (Domains Bacteria & Archaea) KEY POINTS 1. Decomposers: recycle organic and inorganic molecules in environment; makes them available to other organisms. 2. Essential components of symbioses. 3. Encompasses the origins of metabolism and metabolic diversity. 4. Origin of photosynthesis and formation of atmospheric Oxygen Ceno- Meso- zoic zoic ANTIQUITY Humans Paleozoic Colonization of land Animals Origin of solar system and Earth • >3.5 BILLION years old. • Alone for 2 1 4 billion years Proterozoic Archaean Prokaryotes Billions of 2 years ago3 Multicellular eukaryotes Single-celled eukaryotes Atmospheric oxygen General characteristics 1. Small: compare to 10-100µm for 0.5-5µm eukaryotic cell; single-celled; may form colonies. 2. Lack membrane- enclosed organelles. 3. Cell wall present, but different from plant cell wall. 1 2/4/15 General characteristics 4. Occur everywhere, most numerous organisms. – More individuals in a handful of soil then there are people that have ever lived. – By far more individuals in our gut than eukaryotic cells that are actually us. General characteristics 5. Metabolic diversity established nutritional modes of eukaryotes. General characteristics 6. Important decomposers and recyclers 2 2/4/15 General characteristics 6. Important decomposers and recyclers • Form the basis of global nutrient cycles. General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • About 1/2 of all human diseases are caused by Bacteria General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • Extremely important in agriculture as well. Pierce’s disease is caused by Xylella fastidiosa, a Gamma Proteobacteria. It causes over $56 million in damage annually in California. That’s with $34 million spent to control it! = $90 million in California alone. -

The Ligand-Specific Co-Receptor Function of CD44 for Receptor
The ligand-specific co-receptor function of CD44 for receptor tyrosine kinases Zur Erlangung des akademischen Grades eines DOKTORS DER NATURWISSENSCHAFTEN (Dr. rer. nat.) Fakultät für Chemie und Biowissenschaften Karlsruher Institut für Technologie (KIT) - Universitätsbereich genehmigte DISSERTATION von Christian Jung aus Karlsruhe Dekan: Prof. Dr. Martin Bastmeyer Referent: PD Dr. Véronique Orian-Rousseau Korreferent: Prof. Dr. Doris Wedlich Tag der mündlichen Prüfung: 18.4.2012 I Ich versichere, dass ich meine Arbeit selbständig angefertigt und keine anderen als die angegebenen Quellen und Hilfsmittel benutzt, sowie die wörtlich oder inhaltlich übernommenen Stellen als solche kenntlich gemacht und die Satzung der Universität Karlsruhe (TH) zur Sicherung guter wissenschaftlicher Praxis in der jeweils gültigen Fassung beachtet habe. Christian Jung, März 2012 II Zusammenfassung Listeria monocytogenes, ein gram-positives Bakterium, verursacht die Krankheit Listeriose. Eine Möglichkeit, wie L.monocytogenes Wirbeltierzellen infizieren kann, ist das Binden des Bakteriums an die Rezeportyrosinkinase (RTK) Met auf der Wirtszelle durch das bakterielle Protein InlB. Dieses Binden führt zur Aktivierung von Met und schließlich zur Aufnahme in die Zelle. Der erste Teil meine Doktorarbeit zeigt, dass die Infektion von nicht-phagozytotischen Zellen mittels InlB zusätzlich vom Ko-Rezeptor CD44v6 abhängig ist. Desweiteren kann diese bakterielle Infektion mit einem CD44v6-Peptid blockiert werden. Zusätzlich zu der Ko-Rezeptorfunktion von CD44v6 für InlB und Met, die ich gezeigt habe, wurde CD44v6 bereits als Ko-Rezeptor für die Induktion von Met und VEGFR- 2 durch ihre authentischen Liganden HGF and VEGF-A identifiziert. Im zweiten und Hauptteil meiner Doktorarbeit habe ich untersucht, ob diese Ko-Rezeptorfunktion von CD44v6 spezifisch von den Liganden, den Rezeptoren oder beiden bestimmt wird. -

A Generic Large-Scale Cause for Platelet Dysfunction and Depletion in Infection
Published online: 2020-04-12 THIEME 302 A Champion of Host Defense: A Generic Large-Scale Cause for Platelet Dysfunction and Depletion in Infection Martin J. Page, BSc (Hons)1 Etheresia Pretorius, PhD1 1 Department of Physiological Sciences, Stellenbosch University, Address for correspondence Etheresia Pretorius, PhD, Department of Stellenbosch, South Africa Physiological Sciences, Faculty of Science, Stellenbosch University, Private Bag X1 Matieland, Stellenbosch, 7602, South Africa Semin Thromb Hemost 2020;46:302–319. (e-mail: [email protected]). Abstract Thrombocytopenia is commonly associated with sepsis and infections, which in turn are characterized by a profound immune reaction to the invading pathogen. Platelets are one of the cellular entities that exert considerable immune, antibacterial, and antiviral actions, and are therefore active participants in the host response. Platelets are sensitive to surrounding inflammatory stimuli and contribute to the immune response by multiple mechanisms, including endowing the endothelium with a proinflammatory phenotype, enhancing and amplifying leukocyte recruitment and inflammation, promoting the effector functions of immune cells, and ensuring an optimal adaptive immune response. During infection, pathogens and their products influence the platelet response and can even be toxic. However, platelets are able to sense and engage bacteria and viruses to assist in their removal and destruction. Platelets greatly contribute to host defense by multiple mechanisms, including forming immune complexes and aggregates, shedding their granular content, and internalizing pathogens and subsequently being marked for removal. These processes, and the nature of platelet function in general, cause the platelet to be irreversibly consumed in Keywords the execution of its duty. An exaggerated systemic inflammatory response to infection ► platelets can drive platelet dysfunction, where platelets are inappropriately activated and face ► virus immunological destruction. -

In Vivo Dual RNA-Seq Analysis Reveals the Basis for Differential Tissue Tropism of Clinical Isolates of Streptococcus Pneumoniae
In Vivo Dual RNA-Seq Analysis Reveals the Basis for Differential Tissue Tropism of Clinical Isolates of Streptococcus pneumoniae Vikrant Minhas,1,4 Rieza Aprianto,2,4 Lauren J. McAllister,1 Hui Wang,1 Shannon C. David,1 Kimberley T. McLean,1 Iain Comerford,3 Shaun R. McColl,3 James C. Paton,1,5,6,* Jan-Willem Veening,2,5 and Claudia Trappetti,1,5 Supplementary Information Supplementary Table 1. Pneumococcal differential gene expression in the lungs 6 h post-infection, 9-47-Ear vs 9-47M. Genes with fold change (FC) greater than 2 and p < 0.05 are shown. FC values highlighted in blue = upregulated in 9-47-Ear, while values highlighted in red = upregulated in 9- 47M. Locus tag in 9-47- Product padj FC Ear Sp947_chr_00844 Sialidase B 3.08E-10 313.9807 Sp947_chr_02077 hypothetical protein 4.46E-10 306.9412 Sp947_chr_00842 Sodium/glucose cotransporter 2.22E-09 243.4822 Sp947_chr_00841 N-acetylneuraminate lyase 4.53E-09 227.7963 scyllo-inositol 2-dehydrogenase Sp947_chr_00845 (NAD(+)) 4.36E-09 221.051 Sp947_chr_00848 hypothetical protein 1.19E-08 202.7867 V-type sodium ATPase catalytic subunit Sp947_chr_00853 A 1.29E-06 100.5411 Sp947_chr_00846 Beta-glucoside kinase 3.42E-06 98.18951 Sp947_chr_00855 V-type sodium ATPase subunit D 8.34E-06 85.94879 Sp947_chr_00851 V-type sodium ATPase subunit C 2.50E-05 72.46612 Sp947_chr_00843 hypothetical protein 2.17E-05 65.97758 Sp947_chr_00839 HTH-type transcriptional regulator RpiR 3.09E-05 61.28171 Sp947_chr_00854 V-type sodium ATPase subunit B 1.32E-06 50.86992 Sp947_chr_00120 hypothetical protein 3.00E-04 -

Investigation of Peptidyl-Prolyl Cis/Trans Isomerases in the Virulence of Staphylococcus
Investigation of Peptidyl-prolyl cis/trans isomerases in the virulence of Staphylococcus aureus A Dissertation presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Doctor of Philosophy Rebecca A. Keogh August 2020 © 2020 Rebecca A. Keogh. All Rights Reserved. 2 This Dissertation titled Investigation of Peptidyl-prolyl cis/trans isomerases in the virulence of Staphylococcus aureus by REBECCA A. KEOGH has been approved for the Department of Biological Sciences and the College of Arts and Sciences by Ronan K. Carroll Assistant Professor of Biological Sciences Florenz Plassmann Dean, College of Arts and Sciences 3 ABSTRACT REBECCA A. KEOGH, Doctorate of Philosophy, August 2020, Biological Sciences Investigation of peptidyl-prolyl cis/trans isomerases in the virulence of Staphylococcus aureus Director of Dissertation: Ronan K. Carroll Staphylococcus aureus is a leading cause of both hospital and community- associated infections that can manifest in a wide range of diseases. These diseases range in severity from minor skin and soft tissue infections to life-threatening sepsis, endocarditis and meningitis. Of rising concern is the prevalence of antibiotic resistant S. aureus strains in the population, and the lack of new antibiotics being developed to treat them. A greater understanding of the ability of S. aureus to cause infection is crucial to better inform treatments and combat these antibiotic resistant superbugs. The ability of S. aureus to cause such diverse infections can be attributed to the arsenal of virulence factors produced by the bacterium that work to both evade the human immune system and assist in pathogenesis. -

Killing of Gram-Negative Bacteria by Lactoferrin and Lysozyme
Killing of gram-negative bacteria by lactoferrin and lysozyme. R T Ellison 3rd, T J Giehl J Clin Invest. 1991;88(4):1080-1091. https://doi.org/10.1172/JCI115407. Research Article Although lactoferrin has antimicrobial activity, its mechanism of action is not full defined. Recently we have shown that the protein alters the Gram-negative outer membrane. As this membrane protects Gram-negative cells from lysozyme, we have studied whether lactoferrin's membrane effect could enhance the antibacterial activity of lysozyme. We have found that while each protein alone is bacteriostatic, together they can be bactericidal for strains of V. cholerae, S. typhimurium, and E. coli. The bactericidal effect is dose dependent, blocked by iron saturation of lactoferrin, and inhibited by high calcium levels, although lactoferrin does not chelate calcium. Using differing media, the effect of lactoferrin and lysozyme can be partially or completely inhibited; the degree of inhibition correlating with media osmolarity. Transmission electron microscopy shows that E. coli cells exposed to lactoferrin and lysozyme at 40 mOsm become enlarged and hypodense, suggesting killing through osmotic damage. Dialysis chamber studies indicate that bacterial killing requires direct contact with lactoferrin, and work with purified LPS suggests that this relates to direct LPS-binding by the protein. As lactoferrin and lysozyme are present together in high levels in mucosal secretions and neutrophil granules, it is probable that their interaction contributes to host defense. Find the latest version: https://jci.me/115407/pdf Killing of Gram-negative Bacteria by Lactofernn and Lysozyme Richard T. Ellison III*" and Theodore J. Giehl *Medical and tResearch Services, Department of Veterans Affairs Medical Center, and Division ofInfectious Diseases, Department ofMedicine, University ofColorado School ofMedicine, Denver, Colorado 80220 Abstract (4). -

Novel Therapeutic Interventions Towards Improved Management of Septic Arthritis Jian Wang1* and Liucai Wang2
Wang and Wang BMC Musculoskeletal Disorders (2021) 22:530 https://doi.org/10.1186/s12891-021-04383-6 REVIEW Open Access Novel therapeutic interventions towards improved management of septic arthritis Jian Wang1* and Liucai Wang2 Abstract Septic arthritis (SA) represents a medical emergency that needs immediate diagnosis and urgent treatment. Despite aggressive treatment and rapid diagnosis of the causative agent, the mortality and lifelong disability, associated with septic arthritis remain high as close to 11%. Moreover, with the rise in drug resistance, the rates of failure of conventional antibiotic therapy have also increased. Among the etiological agents frequently isolated from cases of septic arthritis, Staphylococcus aureus emerges as a dominating pathogen, and to worsen, the rise in methicillin- resistant S. aureus (MRSA) isolates in bone and joint infections is worrisome. MRSA associated cases of septic arthritis exhibit higher mortality, longer hospital stay, and higher treatment failure with poorer clinical outcomes as compared to cases caused by the sensitive strain i.e methicillin-sensitive S. aureus (MSSA). In addition to this, equal or even greater damage is imposed by the exacerbated immune response mounted by the patient’s body in a futile attempt to eradicate the bacteria. The antibiotic therapy may not be sufficient enough to control the progression of damage to the joint involved thus, adding to higher mortality and disability rates despite the prompt and timely start of treatment. This situation implies that efforts and focus towards studying/ understanding new strategies for improved management of sepsis arthritis is prudent and worth exploring. The review article aims to give a complete insight into the new therapeutic approaches studied by workers lately in this field. -
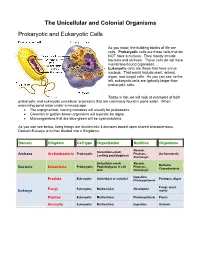
The Unicellular and Colonial Organisms Prokaryotic And
The Unicellular and Colonial Organisms Prokaryotic and Eukaryotic Cells As you know, the building blocks of life are cells. Prokaryotic cells are those cells that do NOT have a nucleus. They mostly include bacteria and archaea. These cells do not have membrane-bound organelles. Eukaryotic cells are those that have a true nucleus. That would include plant, animal, algae, and fungal cells. As you can see, to the left, eukaryotic cells are typically larger than prokaryotic cells. Today in lab, we will look at examples of both prokaryotic and eukaryotic unicellular organisms that are commonly found in pond water. When examining pond water under a microscope… The unpigmented, moving microbes will usually be protozoans. Greenish or golden-brown organisms will typically be algae. Microorganisms that are blue-green will be cyanobacteria. As you can see below, living things are divided into 3 domains based upon shared characteristics. Domain Eukarya is further divided into 4 Kingdoms. Domain Kingdom Cell type Organization Nutrition Organisms Absorb, Unicellular-small; Prokaryotic Photsyn., Archaeacteria Archaea Archaebacteria Lacking peptidoglycan Chemosyn. Unicellular-small; Absorb, Bacteria, Prokaryotic Peptidoglycan in cell Photsyn., Bacteria Eubacteria Cyanobacteria wall Chemosyn. Ingestion, Eukaryotic Unicellular or colonial Protozoa, Algae Protista Photosynthesis Fungi, yeast, Fungi Eukaryotic Multicellular Absorption Eukarya molds Plantae Eukaryotic Multicellular Photosynthesis Plants Animalia Eukaryotic Multicellular Ingestion Animals Prokaryotic Organisms – the archaea, non-photosynthetic bacteria, and cyanobacteria Archaea - Microorganisms that resemble bacteria, but are different from them in certain aspects. Archaea cell walls do not include the macromolecule peptidoglycan, which is always found in the cell walls of bacteria. Archaea usually live in extreme, often very hot or salty environments, such as hot mineral springs or deep-sea hydrothermal vents. -

2011/109440 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date _ . 9 September 2011 (09.09.2011) 2011/109440 Al (51) International Patent Classification: [CH/CH]; Chemin Des Chevreuils 1, 1188 Gimel (CH). C12Q 1/68 (2006.01) G01N 33/53 (2006.01) HOLTERMAN, Daniel [US/US]; 14465 North 14th St., Phoenix, AZ 85022 (US). (21) International Application Number: PCT/US201 1/026750 (74) Agent: AKHAVAN, Ramin; Caris Life Sciences, Inc., 6655 N. MacArthur Blvd., Irving, TX 75039 (US). (22) International Filing Date: 1 March 201 1 (01 .03.201 1) (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, English (25) Filing Language: AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (26) Publication Language: English CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (30) Priority Data: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 61/274,124 1 March 2010 (01 .03.2010) US KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 61/357,5 17 22 June 2010 (22.06.2010) US ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, 61/364,785 15 July 2010 (15.07.2010) us NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (71) Applicant (for all designated States except US): CARIS SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, LIFE SCIENCES LUXEMBOURG HOLDINGS [LU/ TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Signal Transduction, Quorum-Sensing, and Extracellular Protease Activity in Enterococcus Faecalis Biofilm Formation
Signal Transduction, Quorum-Sensing, and Extracellular Protease Activity in Enterococcus faecalis Biofilm Formation The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Carniol, K., and M. S. Gilmore. 2004. Signal Transduction, Quorum- Sensing, and Extracellular Protease Activity in Enterococcus Faecalis Biofilm Formation. Journal of Bacteriology 186, no. 24: 8161–8163. doi:10.1128/jb.186.24.8161-8163.2004. Published Version doi:10.1128/JB.186.24.8161-8163.2004 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:33867369 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA JOURNAL OF BACTERIOLOGY, Dec. 2004, p. 8161–8163 Vol. 186, No. 24 0021-9193/04/$08.00ϩ0 DOI: 10.1128/JB.186.24.8161–8163.2004 Copyright © 2004, American Society for Microbiology. All Rights Reserved. GUEST COMMENTARY Signal Transduction, Quorum-Sensing, and Extracellular Protease Activity in Enterococcus faecalis Biofilm Formation Karen Carniol1,2 and Michael S. Gilmore1,2* Department of Ophthalmology, Harvard Medical School,1 and The Schepens Eye Research Institute,2 Boston, Massachusetts Biofilms are surface-attached communities of bacteria, en- sponse regulator proteins (10). Only one of the mutants gen- cased in an extracellular matrix of secreted proteins, carbohy- erated, fsrA, impaired the ability of E. faecalis strain V583A to drates, and/or DNA, that assume phenotypes distinct from form biofilms in vitro. -

Host and Bacterial Determinants of Staphylococcus Aureus Nasal Colonization in Humans
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2014 Host and Bacterial Determinants of Staphylococcus aureus Nasal Colonization in Humans Gowrishankar Muthukrishnan University of Central Florida Part of the Medical Sciences Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Muthukrishnan, Gowrishankar, "Host and Bacterial Determinants of Staphylococcus aureus Nasal Colonization in Humans" (2014). Electronic Theses and Dissertations, 2004-2019. 1289. https://stars.library.ucf.edu/etd/1289 HOST AND BACTERIAL DETERMINANTS OF STAPHYLOCOCCUS AUREUS NASAL COLONIZATION IN HUMANS by GOWRISHANKAR MUTHUKRISHNAN M.E. Birla Institute of Technology and Science, Pilani, India, 2007 M.S. University of Central Florida, United States of America, 2010 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Burnett School of Biomedical Sciences in the College of Medicine at the University of Central Florida Orlando, Florida Summer Term 2014 Major Professor: Alexander M. Cole © 2014 Gowrishankar Muthukrishnan ii ABSTRACT Staphylococcus aureus (SA), an opportunistic pathogen colonizing the anterior nares in approximately 30% of the human population, causes severe hospital-associated and community-acquired infections. SA nasal carriage plays a critical role in the pathogenesis of staphylococcal infections and SA eradication from the nares has proven to be effective in reducing endogenous infections. -

Structural Basis of Mammalian Mucin Processing by the Human Gut O
ARTICLE https://doi.org/10.1038/s41467-020-18696-y OPEN Structural basis of mammalian mucin processing by the human gut O-glycopeptidase OgpA from Akkermansia muciniphila ✉ ✉ Beatriz Trastoy 1,4, Andreas Naegeli2,4, Itxaso Anso 1,4, Jonathan Sjögren 2 & Marcelo E. Guerin 1,3 Akkermansia muciniphila is a mucin-degrading bacterium commonly found in the human gut that promotes a beneficial effect on health, likely based on the regulation of mucus thickness 1234567890():,; and gut barrier integrity, but also on the modulation of the immune system. In this work, we focus in OgpA from A. muciniphila,anO-glycopeptidase that exclusively hydrolyzes the peptide bond N-terminal to serine or threonine residues substituted with an O-glycan. We determine the high-resolution X-ray crystal structures of the unliganded form of OgpA, the complex with the glycodrosocin O-glycopeptide substrate and its product, providing a comprehensive set of snapshots of the enzyme along the catalytic cycle. In combination with O-glycopeptide chemistry, enzyme kinetics, and computational methods we unveil the molecular mechanism of O-glycan recognition and specificity for OgpA. The data also con- tribute to understanding how A. muciniphila processes mucins in the gut, as well as analysis of post-translational O-glycosylation events in proteins. 1 Structural Biology Unit, Center for Cooperative Research in Biosciences (CIC bioGUNE), Basque Research and Technology Alliance (BRTA), Bizkaia Technology Park, Building 801A, 48160 Derio, Spain. 2 Genovis AB, Box 790, 22007 Lund, Sweden. 3 IKERBASQUE, Basque Foundation for Science, 48013 ✉ Bilbao, Spain. 4These authors contributed equally: Beatriz Trastoy, Andreas Naegeli, Itxaso Anso.