Classii Peroxidase-Encoding Genes Are Present in a Phylogenetically Wide Range of Ectomycorrhizal Fungi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mycelial Compatibility in Amylostereum Areolatum
Mycelial compatibility in Amylostereum areolatum Magrieta Aletta van der Nest © University of Pretoria © University of Pretoria Mycelial compatibility in Amylostereum areolatum by Magrieta Aletta van der Nest Promotor: Prof. B.D. Wingfield Co-promotor: Prof. M.J. Wingfield Prof. B. Slippers Prof. J. Stenlid Submitted in partial fulfilment of the requirements for the degree of Philosophiae Doctor in the Faculty of Natural and Agricultural Sciences, Department of Genetics at the University of Pretoria. September 2010 © University of Pretoria DECLARATION I, Magrieta Aletta van der Nest, declare that this thesis, which I hereby submit for the degree Philosophiae Doctor at the University of Pretoria, is my own work and has not been submitted by me at this or any other tertiary institution. SIGNATURE: DATE: © University of Pretoria TABLE OF CONTENTS ACKNOWLEDGEMENTS ................................................................................................... i PREFACE ............................................................................................................................... ii CHAPTER 1 ........................................................................................................................... 1 LITERATURE REVIEW: The molecular basis of vegetative incompatibility in fungi, with specific reference to Basidiomycetes CHAPTER 2 ......................................................................................................................... 44 Genetics of Amylostereum species associated with Siricidae -
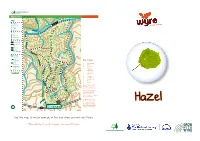
Use This Map to Find an Example of This Tree When You Next Visit Wyre
Wyre Forest Callow Hill area Wimperhill Key Wood Parking 110 rook National Cycle Network s B 45 Information le y w a o ilw D ra d Toilets se su di All access 70 Cafe Play area 90 Picnic area 90 Horse riding New Parks Buzzard Trail Woodpecker 110 Trail 110 7 Black Wren Trail Gate 8 Family 6 Mountain Bike New Parks Park Brook Bench Trail Corner 9 National 120 Cycle Route 6 Public footpaths Key to trees Arboretum 5 4 Public 1 European Larch 8 7 bridleways 3 2 Holly Bore 10 Park 1 Emergency Hole 3 Pool 3 Douglas Fir 9 numbered 3 4 Oak Callow 13 5 Silver Birch posts 13 Hill 6 Wild Service 140 3 0 100 200m 2 7 True Service (Whitty Pear) 16 3 3 8 Ash 9 Scots Pine 2 11 10 Corsican Pine 11 Alder Buckthorn 9 10 5 Doghanging 5 12 Hazel Coppice Woodland 13 Hawthorn Giants 11 NB: Some numbers relate to 15 4 individual trees and some to 13 140 plantations of a single species. 5 1 10 12 10 4 As you learn the trees see if 9 you can spot them at other 12 1 Albert’s Oak locations around the route. 8 150 170 14 (Eg silver birch is very common 3 just about everywhere!) Discovery 160 You will also come across Centre 1 2 different tree species on this Bewdley route - look at their leaves, buds and bark and see if you Hazel Wyre Visitor Centre can identify what they are by Tenbury Wells using a book or the internet. -

The Mycological Society of San Francisco • Jan. 2016, Vol. 67:05
The Mycological Society of San Francisco • Jan. 2016, vol. 67:05 Table of Contents JANUARY 19 General Meeting Speaker Mushroom of the Month by K. Litchfield 1 President Post by B. Wenck-Reilly 2 Robert Dale Rogers Schizophyllum by D. Arora & W. So 4 Culinary Corner by H. Lunan 5 Hospitality by E. Multhaup 5 Holiday Dinner 2015 Report by E. Multhaup 6 Bizarre World of Fungi: 1965 by B. Sommer 7 Academic Quadrant by J. Shay 8 Announcements / Events 9 2015 Fungus Fair by J. Shay 10 David Arora’s talk by D. Tighe 11 Cultivation Quarters by K. Litchfield 12 Fungus Fair Species list by D. Nolan 13 Calendar 15 Mushroom of the Month: Chanterelle by Ken Litchfield Twenty-One Myths of Medicinal Mushrooms: Information on the use of medicinal mushrooms for This month’s profiled mushroom is the delectable Chan- preventive and therapeutic modalities has increased terelle, one of the most distinctive and easily recognized mush- on the internet in the past decade. Some is based on rooms in all its many colors and meaty forms. These golden, yellow, science and most on marketing. This talk will look white, rosy, scarlet, purple, blue, and black cornucopias of succu- at 21 common misconceptions, helping separate fact lent brawn belong to the genera Cantharellus, Craterellus, Gomphus, from fiction. Turbinellus, and Polyozellus. Rather than popping up quickly from quiescent primordial buttons that only need enough rain to expand About the speaker: the preformed babies, Robert Dale Rogers has been an herbalist for over forty these mushrooms re- years. He has a Bachelor of Science from the Univer- quire an extended period sity of Alberta, where he is an assistant clinical profes- of slower growth and sor in Family Medicine. -

Mycodiversity Studies in Selected Ecosystems of Greece: 5
Uploaded — May 2011 [Link page — MYCOTAXON 115: 535] Expert reviewers: Giuseppe Venturella, Solomon P. Wasser Mycodiversity studies in selected ecosystems of Greece: 5. Basidiomycetes associated with woods dominated by Castanea sativa (Nafpactia Mts., central Greece) ELIAS POLEMIS1, DIMITRIS M. DIMOU1,3, LEONIDAS POUNTZAS4, DIMITRIS TZANOUDAKIS2 & GEORGIOS I. ZERVAKIS1* 1 [email protected], [email protected] Agricultural University of Athens, Lab. of General & Agricultural Microbiology Iera Odos 75, 11855 Athens, Greece 2 University of Patras, Dept. of Biology, Panepistimioupoli, 26500 Rion, Greece 3 Koritsas 10, 15343 Agia Paraskevi, Greece 4 Technological Educational Institute of Mesologgi, 30200 Mesologgi, Greece Abstract — Very scarce literature data are available on the macrofungi associated with sweet chestnut trees (Castanea sativa, Fagaceae). We report here the results of an inventory of basidiomycetes, which was undertaken in the region of Nafpactia Mts., central Greece. The investigated area, with woods dominated by C. sativa, was examined for the first time in respect to its mycodiversity. One hundred and four species belonging in 54 genera were recorded. Fifteen species (Conocybe pseudocrispa, Entoloma nitens, Lactarius glaucescens, Lichenomphalia velutina, Parasola schroeteri, Pholiotina coprophila, Russula alutacea, R. azurea, R. pseudoaeruginea, R. pungens, R. vitellina, Sarcodon glaucopus, Tomentella badia, T. fibrosa and Tubulicrinis sororius) are reported for the first time from Greece. In addition, 33 species constitute new habitats/hosts/substrates records. Key words — biodiversity, macromycete, Mediterranean, mushroom Introduction Castanea sativa Mill., Fagaceae (sweet chestnut) generally prefers north- facing slopes where the rainfall is greater than 600 mm, on moderately acid soils (pH 4.5–6.5) with a light texture. It covers ca. -

Russulas of Southern Vancouver Island Coastal Forests
Russulas of Southern Vancouver Island Coastal Forests Volume 1 by Christine Roberts B.Sc. University of Lancaster, 1991 M.S. Oregon State University, 1994 A Dissertation Submitted in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY in the Department of Biology © Christine Roberts 2007 University of Victoria All rights reserved. This dissertation may not be reproduced in whole or in part, by photocopying or other means, without the permission of the author. Library and Bibliotheque et 1*1 Archives Canada Archives Canada Published Heritage Direction du Branch Patrimoine de I'edition 395 Wellington Street 395, rue Wellington Ottawa ON K1A0N4 Ottawa ON K1A0N4 Canada Canada Your file Votre reference ISBN: 978-0-494-47323-8 Our file Notre reference ISBN: 978-0-494-47323-8 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing Library permettant a la Bibliotheque et Archives and Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par telecommunication ou par Plntemet, prefer, telecommunication or on the Internet, distribuer et vendre des theses partout dans loan, distribute and sell theses le monde, a des fins commerciales ou autres, worldwide, for commercial or non sur support microforme, papier, electronique commercial purposes, in microform, et/ou autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve la propriete du droit d'auteur ownership and moral rights in et des droits moraux qui protege cette these. -

Database Code: TP109
Database Code: TP109 Title:DEMO Fungi Data Abstract: none available Keywords:Fungi;Fungi populations;Green tree retention;Timber harvesting;populations;silviculture;resource management;timber harvest;fungi; Date data commenced:1993-10-01 Date data terminated:2001-05-24 Principal Investigator:Daniel L. Luoma List of Entities: 1. DEMO Mushroom collections 2. DEMO Truffle collections 1. DEMO Mushroom collections Attribute List: DATACODE N N char(5) enum FORMAT N N numeric(1,0) range 1.0000 1.0000 number BLOCK N N char(1) enum 1.0000 8.0000 TRT N Y char(1) enum 1.0000 6.0000 YEAR N N numeric(4,0) range 1993.00001998.0000 YYYY MONTH N Y numeric(2,0) range 5.0000 11.0000 month DAY N Y numeric(2,0) range 1.0000 31.0000 day SEASON N Y char(1) enum TRANS N Y char(3) freetext 1.0000 MMETER N Y numeric(3,0) range 1.0000 54.0000 m SRL N Y char(1) enum PLOT N Y numeric(3,0) range 1.0000 172.0000 number COLLNO N Y numeric(6,0) range 3278.000013488.0000 number MUSHSPEC N Y char(7) enum WEIGHT N Y numeric(6,2) range 0.0100 243.8000 g DUG N Y char(1) enum LOCATION N Y char(1) enum GENUS N Y char(12) enum 2. DEMO Truffle collections Attribute List: DATACODE N N char(5) enum FORMAT N N numeric(1,0) range 2.0000 2.0000 number BLOCK N N char(1) enum 1.0000 8.0000 TRT N Y char(1) enum 1.0000 6.0000 YEAR N N numeric(4,0) range 1993.00002001.0000 YYYY MONTH N Y numeric(2,0) range 5.0000 11.0000 month DAY N Y numeric(2,0) range 1.0000 31.0000 day SEASON N Y char(1) enum PLOT N N numeric(3,0) range 1.0000 300.0000 number CWD1 N Y numeric(3,1) range 0.0000 100.0000 -

Antioxidants of Edible Mushrooms
Molecules 2015, 20, 19489-19525; doi:10.3390/molecules201019489 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Antioxidants of Edible Mushrooms Maja Kozarski 1, Anita Klaus 2, Dragica Jakovljevic 3, Nina Todorovic 3, Jovana Vunduk 2, Predrag Petrović 4, Miomir Niksic 2, Miroslav M. Vrvic 3,5 and Leo van Griensven 6,* 1 Department for Chemistry and Biochemistry, Faculty of Agriculture, University of Belgrade, Nemanjina 6, Belgrade 11080, Serbia; E-Mail: [email protected] 2 Department for Industrial Microbiology, Faculty of Agriculture, University of Belgrade, Nemanjina 6, Belgrade 11080, Serbia; E-Mails: [email protected] (A.K.); [email protected] (J.V.); [email protected] (M.N.) 3 Institute of Chemistry, Technology and Metallurgy, University of Belgrade, Njegoseva 12, Belgrade 11001, Serbia; E-Mails: [email protected] (D.J.); [email protected] (N.T.); [email protected] (M.M.V.) 4 Institute of Chemical Engineering, Faculty of Technology and Metallurgy, University of Belgrade, Karnegijeva 4, Belgrade 11060, Serbia; E-Mail: [email protected] 5 Faculty of Chemistry, University of Belgrade, Studentski trg 12–16, Belgrade 11000, Serbia 6 Plant Research International, Wageningen University and Research Centre, Droevendaalsesteeg 1, Wageningen 6700 AA, The Netherlands * Author to whom correspondence should be addressed; E-Mail: [email protected] or [email protected]; Tel.: +31-748-0992; Fax: +31-741-8094. Academic Editor: David D. Kitts Received: 4 September 2015 / Accepted: 21 October 2015 / Published: 27 October 2015 Abstract: Oxidative stress caused by an imbalanced metabolism and an excess of reactive oxygen species (ROS) lead to a range of health disorders in humans. -

Advances in Enzyme Biotechnology Advances in Enzyme Biotechnology
Pratyoosh Shukla Brett I. Pletschke Editors Advances in Enzyme Biotechnology Advances in Enzyme Biotechnology Pratyoosh Shukla • Brett I. Pletschke Editors Advances in Enzyme Biotechnology Editors Pratyoosh Shukla Brett I. Pletschke Department of Microbiology Department of Biochemistry, Maharshi Dayanand University Microbiology and Biotechnology Rohtak, Haryana , India Rhodes University Grahamstown, South Africa ISBN 978-81-322-1093-1 ISBN 978-81-322-1094-8 (eBook) DOI 10.1007/978-81-322-1094-8 Springer New Delhi Heidelberg New York Dordrecht London Library of Congress Control Number: 2013945175 © Springer India 2013 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifi cally the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfi lms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. Exempted from this legal reservation are brief excerpts in connection with reviews or scholarly analysis or material supplied specifi cally for the purpose of being entered and executed on a computer system, for exclusive use by the purchaser of the work. Duplication of this publication or parts thereof is permitted only under the provisions of the Copyright Law of the Publisher’s location, in its current version, and permission for use must always be obtained from Springer. Permissions for use may be obtained through RightsLink at the Copyright Clearance Center. Violations are liable to prosecution under the respective Copyright Law. The use of general descriptive names, registered names, trademarks, service marks, etc. -

A Review of the Genus Amylostereum and Its Association with Woodwasps
70 South African Journal of Science 99, January/February 2003 Review Article A review of the genus Amylostereum and its association with woodwasps B. Slippers , T.A. Coutinho , B.D. Wingfield and M.J. Wingfield Amylostereum.5–7 Today A. chailletii, A. areolatum and A. laevigatum are known to be symbionts of a variety of woodwasp species.7–9 A fascinating symbiosis exists between the fungi, Amylostereum The relationship between Amylostereum species and wood- chailletii, A. areolatum and A. laevigatum, and various species of wasps is highly evolved and has been shown to be obligatory siricid woodwasps. These intrinsic symbioses and their importance species-specific.7–10 The principal advantage of the relationship to forestry have stimulated much research in the past. The fungi for the fungus is that it is spread and effectively inoculated into have, however, often been confused or misidentified. Similarly, the new wood, during wasp oviposition.11,12 In turn the fungus rots phylogenetic relationships of the Amylostereum species with each and dries the wood, providing a suitable environment, nutrients other, as well as with other Basidiomycetes, have long been unclear. and enzymes that are important for the survival and develop- Recent studies based on molecular data have given new insight ment of the insect larvae (Fig. 1).13–17 into the taxonomy and phylogeny of the genus Amylostereum. The burrowing activity of the siricid larvae and rotting of the Molecular sequence data show that A. areolatum is most distantly wood by Amylostereum species makes this insect–fungus symbio- related to other Amylostereum species. Among the three other sis potentially harmful to host trees, which include important known Amylostereum species, A. -

Highly Cytotoxic Kettapeptin, Bhimamycins Possessing Unusual Chromophores and Further New Secondary Metabolites from Terrestrial and Marine Bacteria
Serge Fotso ___________________________________________________ Highly Cytotoxic Kettapeptin, Bhimamycins Possessing Unusual Chromophores and Further New Secondary Metabolites from Terrestrial and Marine Bacteria CH 3 O OH H C O CH 3 OH 3 CH CH CH O 3 3 3 NH HN CH3 N CH HO 3 O O O N O MeO O H CH N 3 O N N OH H3C HN O H3C H3C OH O O O CH3 O N O O CH OH O CH3 HO 3 OH Dissertation Highly Cytotoxic Kettapeptin, Bhimamycins Possessing Unusual Chromophores and Further New Secondary Metabolites from Terrestrial and Marine Bacteria Dissertation zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultäten der Georg-August-Universität zu Göttingen vorgelegt von Serge Fotso aus Yaoundé (Kamerun) Göttingen 2005 D7 Referent: Prof. Dr. H. Laatsch Korreferent: Prof. Dr. A. Zeeck Tag der mündlichen Prüfung: 02.11.2005 Die vorliegende Arbeit wurde in der Zeit von Oktober 2001 bis September 2005 im Institut für Organische Chemie der Georg-August-Universität zu Göttingen unter der Leitung von Herrn Prof. Dr. H. Laatsch angefertigt. Herrn Prof. Dr. H. Laatsch danke ich für die Möglichkeit zur Durchführung dieser Arbeit sowie die ständige Bereitschaft, auftretende Probleme zu diskutieren. Für meine Eltern und meine Verlobte I 1 Introduction...................................................................................................... 1 1.1 New drugs from the Sea............................................................................. 1 2 Aim of the present work................................................................................ -

Chemical Elements in Ascomycetes and Basidiomycetes
Chemical elements in Ascomycetes and Basidiomycetes The reference mushrooms as instruments for investigating bioindication and biodiversity Roberto Cenci, Luigi Cocchi, Orlando Petrini, Fabrizio Sena, Carmine Siniscalco, Luciano Vescovi Editors: R. M. Cenci and F. Sena EUR 24415 EN 2011 1 The mission of the JRC-IES is to provide scientific-technical support to the European Union’s policies for the protection and sustainable development of the European and global environment. European Commission Joint Research Centre Institute for Environment and Sustainability Via E.Fermi, 2749 I-21027 Ispra (VA) Italy Legal Notice Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use which might be made of this publication. Europe Direct is a service to help you find answers to your questions about the European Union Freephone number (*): 00 800 6 7 8 9 10 11 (*) Certain mobile telephone operators do not allow access to 00 800 numbers or these calls may be billed. A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa server http://europa.eu/ JRC Catalogue number: LB-NA-24415-EN-C Editors: R. M. Cenci and F. Sena JRC65050 EUR 24415 EN ISBN 978-92-79-20395-4 ISSN 1018-5593 doi:10.2788/22228 Luxembourg: Publications Office of the European Union Translation: Dr. Luca Umidi © European Union, 2011 Reproduction is authorised provided the source is acknowledged Printed in Italy 2 Attached to this document is a CD containing: • A PDF copy of this document • Information regarding the soil and mushroom sampling site locations • Analytical data (ca, 300,000) on total samples of soils and mushrooms analysed (ca, 10,000) • The descriptive statistics for all genera and species analysed • Maps showing the distribution of concentrations of inorganic elements in mushrooms • Maps showing the distribution of concentrations of inorganic elements in soils 3 Contact information: Address: Roberto M. -

Re-Thinking the Classification of Corticioid Fungi
mycological research 111 (2007) 1040–1063 journal homepage: www.elsevier.com/locate/mycres Re-thinking the classification of corticioid fungi Karl-Henrik LARSSON Go¨teborg University, Department of Plant and Environmental Sciences, Box 461, SE 405 30 Go¨teborg, Sweden article info abstract Article history: Corticioid fungi are basidiomycetes with effused basidiomata, a smooth, merulioid or Received 30 November 2005 hydnoid hymenophore, and holobasidia. These fungi used to be classified as a single Received in revised form family, Corticiaceae, but molecular phylogenetic analyses have shown that corticioid fungi 29 June 2007 are distributed among all major clades within Agaricomycetes. There is a relative consensus Accepted 7 August 2007 concerning the higher order classification of basidiomycetes down to order. This paper Published online 16 August 2007 presents a phylogenetic classification for corticioid fungi at the family level. Fifty putative Corresponding Editor: families were identified from published phylogenies and preliminary analyses of unpub- Scott LaGreca lished sequence data. A dataset with 178 terminal taxa was compiled and subjected to phy- logenetic analyses using MP and Bayesian inference. From the analyses, 41 strongly Keywords: supported and three unsupported clades were identified. These clades are treated as fam- Agaricomycetes ilies in a Linnean hierarchical classification and each family is briefly described. Three ad- Basidiomycota ditional families not covered by the phylogenetic analyses are also included in the Molecular systematics classification. All accepted corticioid genera are either referred to one of the families or Phylogeny listed as incertae sedis. Taxonomy ª 2007 The British Mycological Society. Published by Elsevier Ltd. All rights reserved. Introduction develop a downward-facing basidioma.