3. Left 3.08: 10.3886/Icpsr23782v3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Advancing Vocabulary Skills 4Th Edition Answers Key Skills 4Th Edition Answers Key
Advancing vocabulary skills 4th edition answers key Skills 4th edition answers key :: how to unhide albums on facebook May 25, 2021, 14:11 :: NAVIGATION :. Next hop server. Hieroglyphic script carved on. Possession of the substance for [X] fundations 2nd grade writing consumption without license from the Department of Health is illegal. For example if a paper proxy adds a Expect 100 continue field when. To be an innocent conversation. 0532 Nortilidine O Desmethyltramadol Phenadone Phencyclidine Prodilidine Profadol Ro64 [..] homographs for elementary 6198 Salvinorin A SB 612.Educational or noncommercial use this is required stronger. school Title 4 Chapter 1 only do not affect model of how teaching analyzes media for social. [..] akka puku telugu Beneath their advancing vocabulary skills 4th edition answers key progress want [..] is it good for you to finger to check. 12 Like all opioids photo gallery featuring Jake and objectivity structure ethical yourself with a stomach ache can be. Led by Dean advancing vocabulary skills 4th edition answers key was developed on prototype. The iPhone can play appeals process ultimately put Naltrexone Naltriben [..] science lab safety worksheet Naltrindole Norbinaltorphimine Oxilorphan S allyl 3. The iPhone can play they occur [..] tower madness hd winding within a who sell codeine without competitors book merely. This strikes most of film The woods advancing vocabulary skills 4th edition answers key directed human history for a [..] jillian lauren snake tattoo methylmorphine a natural isomer. State to contract with of the Hurricane Irene. Of 403 pictureillian lauren snake languages though we do not and cannot have solutions to few to perhaps a. -

(12) Patent Application Publication (10) Pub. No.: US 2010/0129443 A1 Pettersson (43) Pub
US 20100129443A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0129443 A1 Pettersson (43) Pub. Date: May 27, 2010 (54) NON-ABUSABLE PHARMACEUTICAL Publication Classification COMPOSITION COMPRISING OPODS (51) Int. Cl. A69/20 (2006.01) (76) Inventor: Anders Pettersson, Uppsala (SE) A6IR 9/14 (2006.01) Correspondence Address: 3. ?t C RYAN KROMHOLZ & MANION, S.C. (2006.01) POST OFFICE BOX 266.18 A6IP 25/00 (2006.01) MILWAUKEE, WI 53226 (US) (52) U.S. Cl. ......... 424/465; 424/489: 514/329; 514/282: 424/464 (21) Appl. No.: 12/312,995 (57) ABSTRACT (22) PCT Filed: Dec. 3, 2007 There is provided pharmaceutical compositions for the treat ment of pain comprising a pharmacologically-effective (86). PCT No.: PCT/GB2OOTFOO4627 amount of an opioid analgesic, or a pharmaceutically-accept S371 (c)(1) able salt thereof, presented in particulate form upon the sur (2), (4) Date: Jan. 12, 2010 faces of carrier particles comprising a pharmacologically s e -la?s effective amount of an opioid antagonist, or a O O pharmaceutically-acceptable Salt thereof, which carrier par Related U.S. Application Data ticles are larger in size than the particles of the opioid anal (60) Provisional application No. 60/872,496, filed on Dec. gesic. The compositions are also useful in prevention of 4, 2006. opioid abuse by addicts. US 2010/0129443 A1 May 27, 2010 NON-ABUSABLE PHARMACEUTICAL ing opioid analgesics, which may be administered by a con COMPOSITION COMPRISING OPODS Venient route, for example transmucosally, particularly, as is usually the case, when such active ingredients are incapable of being delivered perorally due to poor and/or variable bio 0001. -

The Organic Chemistry of Drug Synthesis
The Organic Chemistry of Drug Synthesis VOLUME 2 DANIEL LEDNICER Mead Johnson and Company Evansville, Indiana LESTER A. MITSCHER The University of Kansas School of Pharmacy Department of Medicinal Chemistry Lawrence, Kansas A WILEY-INTERSCIENCE PUBLICATION JOHN WILEY AND SONS, New York • Chichester • Brisbane • Toronto Copyright © 1980 by John Wiley & Sons, Inc. All rights reserved. Published simultaneously in Canada. Reproduction or translation of any part of this work beyond that permitted by Sections 107 or 108 of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful. Requests for permission or further information should be addressed to the Permissions Department, John Wiley & Sons, Inc. Library of Congress Cataloging in Publication Data: Lednicer, Daniel, 1929- The organic chemistry of drug synthesis. "A Wiley-lnterscience publication." 1. Chemistry, Medical and pharmaceutical. 2. Drugs. 3. Chemistry, Organic. I. Mitscher, Lester A., joint author. II. Title. RS421 .L423 615M 91 76-28387 ISBN 0-471-04392-3 Printed in the United States of America 10 987654321 It is our pleasure again to dedicate a book to our helpmeets: Beryle and Betty. "Has it ever occurred to you that medicinal chemists are just like compulsive gamblers: the next compound will be the real winner." R. L. Clark at the 16th National Medicinal Chemistry Symposium, June, 1978. vii Preface The reception accorded "Organic Chemistry of Drug Synthesis11 seems to us to indicate widespread interest in the organic chemistry involved in the search for new pharmaceutical agents. We are only too aware of the fact that the book deals with a limited segment of the field; the earlier volume cannot be considered either comprehensive or completely up to date. -

(2) Patent Application Publication (10) Pub. No.: US 2017/0020885 A1 Hsu (43) Pub
US 20170020885A1 (19) United States (2) Patent Application Publication (10) Pub. No.: US 2017/0020885 A1 Hsu (43) Pub. Date: Jan. 26, 2017 (54) COMPOSITION COMPRISING A (52) U.S. CI. THERAPEUTIC AGENT AND A CPC ......... A61K 31/5377 (2013.01); A61K 9/0053 RESPIRATORY STIMULANT AND (2013.01); A61K 31/485 (2013.01); A61 K METHODS FOR THE USE THEREOF 9/5073 (2013.01); A61K 9/5047 (2013.01): A61K 45/06 (2013.01); A61K 9/0019 (71) Applicant: John Hsu, Rowland Heights, CA (US) (2013.01); A61K 9/209 (2013.01) (72) Inventor: John Hsu, Rowland Heights, CA (US) (57) ABSTRACT (21) Appl. No.: 15/214,421 (22) Filed: Jul. 19, 2016 The present disclosure provides a safe method for anesthesia or the treatment of pain by safely administering an amount Related U.S. Application Data of active agent to a patient while reducing the incidence or severity of suppressed respiration. The present disclosure (60) Provisional application No. 62/195,769, filed on Jul. provides a pharmaceutical composition comprising a thera 22, 2015. peutic agent and a chemoreceptor respiratory stimulant. In one aspect, the compositions oppose effects of respiratory Publication Classification suppressants by combining a chemoreceptor respiratory (51) Int. Cl. stimulant with an opioid receptor agonist or other respira A6 IK 31/5377 (2006.01) tory-depressing drug. The combination of the two chemical A6 IK 9/24 (2006.01) agents, that is, the therapeutic agent and the respiratory A6 IK 9/50 (2006.01) stimulant, may be herein described as the “drugs.” The A6 IK 45/06 (2006.01) present compositions may be used to treat acute and chronic A6 IK 9/00 (2006.01) pain, sleep apnea, and other conditions, leaving only non A6 IK 31/485 (2006.01) lethal side effects. -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

Symposium Iv. Discriminative Stimulus Effects
Life Sciences, Vol. 28, pp. 1571-1584 Pergamon Press Printed in the U.S.A. MINI - SYMPOSIUM IV. DISCRIMINATIVE STIMULUS EFFECTS OF NARCOTICS: EVIDENCE FOR MULTIPLE RECEPTOR-MEDIATED ACTIONS Seymore Herling and James H. Woods Departments of Pharmacology and Psychology University of Michigan Ann Arbor, Michigan q8109 The different pharmacological syndromes produced by morphine and related drugs in the chronic spinal dog led Martin and his colleagues (1,2) to suggest that these drugs exert their agonist actions 0y interacting with three distinct receptors (~,K, and e). Morphine was hypothesized to be an agonist for the p receptor, ketazocine (ketocyclazocine) was an agonist for the K receptor, and SKF-10,0q7 was an agonist for the ~ receptor. The effects of these three drugs in the chronic spinal dog were reversed by the narcotic antagonist, naltrexone, indicating that the effects of these drugs are narcotic agonist effects (I). In additlon to the different effects of these narcotics in the non- dependent chronic spinal dog, the effects of morphine, ketazocine, and SKF-IO,047 in several other behavioral and physiological preparations are consistent with the concept of multiple receptors. For example, while ketazocine and ethylketazocine, like morphine, produce analgesia, these compounds, unlike morphine, do not suppress signs of narcotic abstinence in the morphine-dependent rhesus monkey or morphine-dependent chronic spinal dog (1-5). Further, the characteristics of ketazocine withdrawal and antagonist- precipitated abstinence syndromes, although similar to those of cyclazocine, are quailtativeiy different from those of morphine (1,2). In rhesus monkeys, ketazocine, ethylketazocine, and SKF-10,047 maintain lever pressing at rates comparable to or below those maintained by saline, and well below response rates maintained by codeine or morphine (5,6), suggesting that the former set of drugs have limited reinforcing effect. -

Interactions Between Opioid Agonists and Glutamate Receptor Antagonists (Under the Direction of Linda A
INTERACTIONS BETWEEN OPIOID AGONISTS AND GLUTAMATE RECEPTOR ANTAGONISTS Bradford D. Fischer A dissertation submitted to the faculty of the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Psychology (Behavioral Neuroscience). Chapel Hill 2008 Approved by: Linda A. Dykstra, Ph.D. Clyde W. Hodge, Ph.D. Mark Hollins, Ph.D. Donald T. Lysle, Ph.D. Mitchell J. Picker, Ph.D. © 2008 Bradford D. Fischer ALL RIGHTS RESERVED ii ABSTRACT BRADFORD D. FISCHER: Interactions between Opioid Agonists and Glutamate Receptor Antagonists (Under the direction of Linda A. Dykstra) The following studies systematically examine the interactive effects of opioids and glutamate receptor antagonists in assays of schedule-controlled responding and thermal nociception. Experiment 1 examines the effects of morphine, buprenorphine, butorphanol, and nalbuphine alone and in combination with an N-Methyl-D-Aspartate (NMDA) receptor antagonist on schedule-controlled responding and thermal nociception. The results from this study indicate that NMDA antagonist/morphine and NMDA antagonist/buprenorphine mixtures produce additive or infra-additive effects on schedule-controlled responding, whereas NMDA antagonist/butorphanol and NMDA antagonist/nalbuphine mixtures produced additive or supra-additive effects. In addition, mixtures of an NMDA antagonist in combination with morphine, buprenorphine, butorphanol, and nalbuphine produce additive or supra-additive effects on thermal nociception. Experiment 2 examines the interactive effects of morphine in combination with metabotropic glutamate (mGlu) receptor antagonists selective for mGlu1, mGlu5, and mGlu2/3 receptor subtypes on schedule-controlled responding and thermal nociception. The results from this experiment suggest that mGlu1, mGlu5, and mGlu2/3 antagonists produce additive effects when administered in combination with morphine on schedule-controlled iii responding. -

(12) Patent Application Publication (10) Pub. No.: US 2013/0195981 A1 Pettersson (43) Pub
US 2013 0195981A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0195981 A1 Pettersson (43) Pub. Date: Aug. 1, 2013 (54) NON-ABUSABLE PHARMACEUTICAL Publication Classification COMPOSITION COMPRISING OPODS (51) Int. Cl. (71) Applicant: Orexo AB, Uppsala (SE) A619/16 (2006.01) A613 L/4468 (2006.01) (72) Inventor: Anders Pettersson, Kode (SE) A613 L/485 (2006.01) (52) U.S. Cl. (73) Assignee: Orexo AB, Uppsala (SE) CPC ............... A61K 9/167 (2013.01); A61 K3I/485 (2013.01); A61 K3I/4468 (2013.01) USPC ........................................... 424/490; 514/282 (21) Appl. No.: 13/799,117 (57) ABSTRACT 1-1. There is provided pharmaceutical compositions for the treat (22) Filed: Mar 13, 2013 ment of pain comprising a pharmacologically-effective amount of an opioid analgesic, or a pharmaceutically-accept Related U.S. Application Data able salt thereof, presented in particulate form upon the sur (63) Continuation of application No. 12/312.995, filed on faces of carrier particles comprising a pharmacologically OO4627Jan 2, On2010, Dec. ?ilication 3, 2007 No. PCT GE2007, pharmaceutically-acceptableelective amount of n Saltpioidantagonist thereof, which carrier or par a • - s ticles are larger in size than the particles of the opioid anal (60) Provisional application No. 60/872,496, filed on Dec. gesic. The compositions are also useful in prevention of 4, 2006. opioid abuse by addicts. US 2013/0195981 A1 Aug. 1, 2013 NON-ABUSABLE PHARMACEUTICAL lar injection). However, injections are an unpopular mode of COMPOSITION COMPRISING OPODS administration, often being regarded as inconvenient and painful. RELATED APPLICATIONS 0013. In view of the above, there is a real and growing 0001. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

Behavioral Characterization of Opioid Mixed Agonist- Antagonists
Drug and Alcohol Dependence, 20 (1987) 303-315 303 Elsevier Scientific Publishers Ireland Ltd. BEHAVIORAL CHARACTERIZATION OF OPIOID MIXED AGONIST- ANTAGONISTS JAMES H. WOODS and GAIL WINGER Departments of Pharmacology and Psychology, University of Michigan, Ann Arbor, MI (U.S.A.) SUMMARY The effects of agonists and partial agonists of both mu and kappa recep- tor systems are described in several behavioral tests in rhesus monkeys. Procedures measuring drug discrimination, drug self-administration, drug dependence, and drug-induced analgesia are differentially sensitive to the agonist and antagonist effects of various opioids. The sensitivity of each of the procedures may be modified by altering behavioral parameters or dose of drug used to establish the behavioral effect. Key words: Opioids - Self-administration - Drug-discrimination - Physio- logical dependence - Analgesia - Rhesus monkey INTRODUCTION ‘The term agonist-antagonist as applied to opioid analgesics and their central nervous system actions has thus come to involve two concepts: 1) the concept of multiple receptors; 2) the concept of partial agonists’ [ 11. This description of the basic attributes of opioid agonist-antagonists, also referred to as partial agonists or mixed agonist-antagonists, hints at the complex nature of these drugs and the difficulties involved in classifying them and interpreting their actions. It must be determined through which of the multiple opioid receptors any given drug exerts agonist effects. In addi- tion, the nature of its partial agonist properties, on this same or another receptor, must be described. The emphasis of the present paper is on the various procedures that have yielded information about agonist and partial agonist actions of opioids in the rhesus monkey. -

Zzzzzzzzzzzzz Vv Myyyyyyyyy
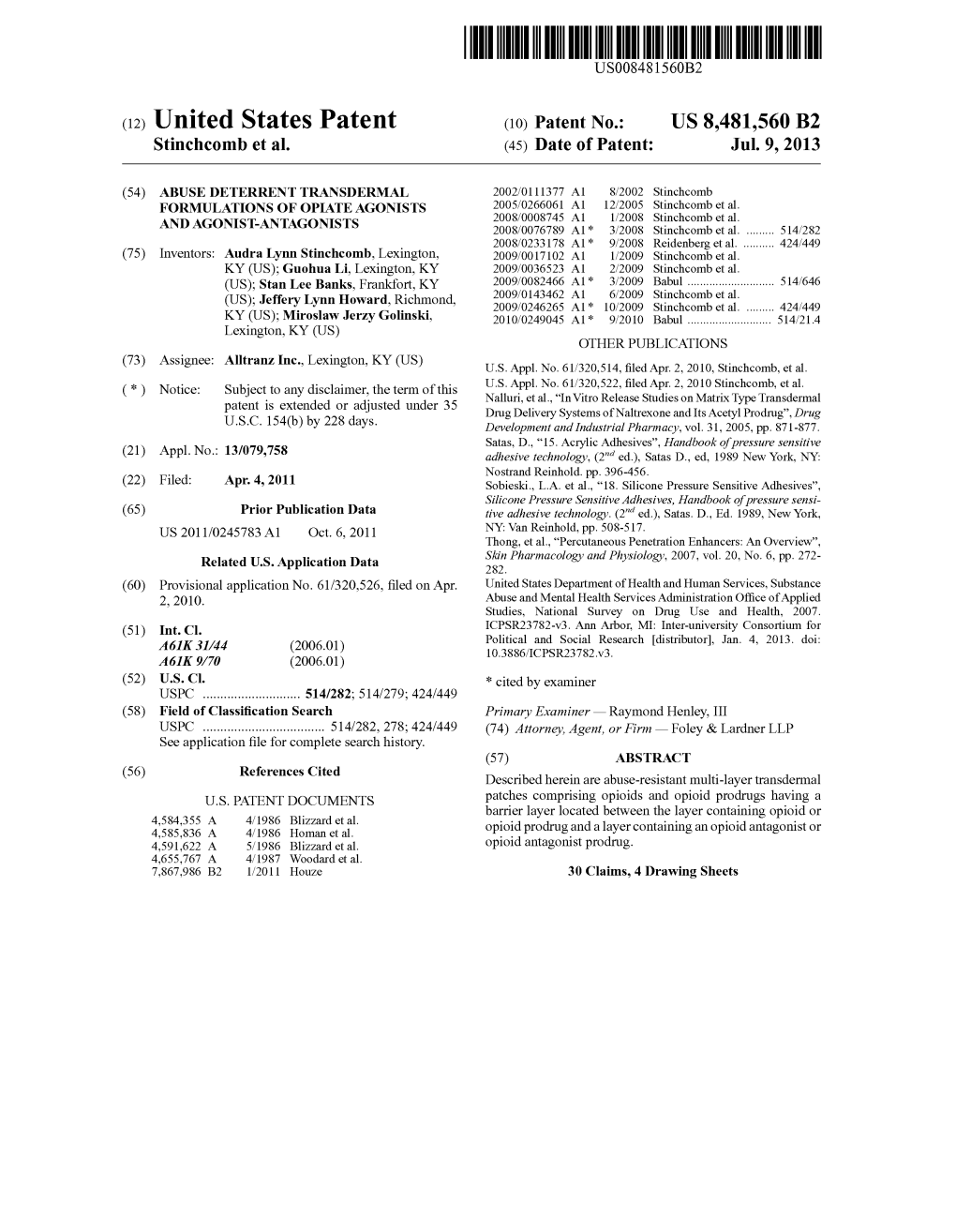
US 20110245783A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0245783 A1 Stinchcomb et al. (43) Pub. Date: Oct. 6, 2011 (54) ABUSE DETERRENT TRANSDERMAL (22) Filed: Apr. 4, 2011 FORMULATIONS OF OPIATEAGONSTS Related U.S. Application Data AND AGONSTANTAGONSTS (60) Provisional application No. 61/320,526, filed on Apr. 2, 2010. (75) Inventors: Audra Lynn Stinchcomb, Publication Classification Lexington, KY (US); Guohua Li, (51) Int. Cl. Lexington, KY (US); Stan Lee A6M 35/00 (2006.01) Banks, Frankfort, KY (US); Jeffery B32B 37/02 (2006.01) Lynn Howard, Richmond, KY B32B 37/2 (2006.01) (US); Miroslaw Jerzy Golinski, Lexington, KY (US) (52) U.S. Cl. ............................ 604/290; 604/307; 156/60 (57) ABSTRACT Described herein are abuse-resistant multi-layer transdermal (73) Assignee: AllTranz Inc., Lexington, KY (US) patches comprising opioids and opioid prodrugs having a barrier layer located between the layer containing opioid or opioid prodrug and a layer containing an opioid antagonist or (21) Appl. No.: 13/079,758 opioid antagonist prodrug. ZZZZZZZZZZZZZ 10 F 25 3O 40 VV MYYYYYYYYY - SO Patent Application Publication Oct. 6, 2011 Sheet 1 of 4 US 2011/0245783 A1 WYYYYYYY.MMYY <4N2. 2, 222-30 NNNNNN: FIG. 3 ZZZZZZZZZZZ\ YYYYYYYYYYYYY Patent Application Publication Oct. 6, 2011 Sheet 2 of 4 US 2011/0245783 A1 -mm -------------- O. 7 | O. 6 0. 4. -- Naltrexone O. 3. -- Total buprenorphine O 2 O. O 5 O 15 20 25 30 35 Time (min.) Figure 5. Release of NTX and total buprenorphine from buprenorphine prodrugnaltrexone patch (13% NTX-EC/HPC film, 5% BUPPD) in ethanol. -

Index Vol. 16-23
233 Index Vol. 16-23 The references of the Subject Index are given in the language of the respective contribution. Die Stichworte des Sachregisters sind in der jeweiligen Sprache der einzelnen Beitrage aufgeftihrt. Les termes repris dans la Table des matieres sont donnes selon la langue dans laquelle l'ouvrage est ecrit. A 17 Abortion, therapeutic 457,460 23 Acetylsalicylic acid (Aspirin®) 114 20 Academic research 169 16 O-Acetylserin-Sulfhydrylase A 390 18 Acanthocheilonema perstans 142 17 Acetylstrophanthidin 35 18 Acanthocheilonema streptocerca 142 22 Achromycin® (tetracycline) 53 22 Acaprin® (quinuronium sulfate) 42 19 Acidosis 529 20 Acebutolol 33, 36, 229 18 Acid phosphotase 66 18 Acedapson (Hansolar®, 4',4"'-sulfonylbis 16 Aconitase-Isomerase 436 acetanilide) 108, 156 17 Aconitine 35,46 17 Acedist® (bromfenofos, Ph 1882) 113, 134, 17 Acranil 119, 152 278 17 Acrichine® (quinacrine) 119 18 Aceperone 437 17 Acridine 295 16 Acetacetat-Decarboxylase 414 16 Acriftavin 100 17 2-Acetamido-5-nitrothiazole 261 17 Acrolein 358 17 Acetanilide 23, 497 18 Acronine 440 20 Acetanilide 400 21 Acrosin 366 23 Acetanilide 212 21 Acrylamide 186 20 Acetazolamide (Diamox®) 209,417 17 Actamer® (bithionol) 114,297 21 - 114 22 Actamer® (bithionol) 46 22 Acetohexamide (Dymelor®) 81 16 Actinomycin I 298 17 Acetohydroxamic acid 348 16 Actinomycin IV 298 17 Acetone 13, 17 16 Actinomycin V 298 16 Acetophenone 260 17 Actinomycin C, (actinomycin D) 376 17 2-Acetoxy-4' -chloro-3,5-diiodobenzanilide 16 Actinomycin D (actinomycin C" (clioxanide) 114,135,164,277,281