Zzzzzzzzzzzzz Vv Myyyyyyyyy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2020 Kansas Statutes
2020 Kansas Statutes 65-4105. Substances included in schedule I. (a) The controlled substances listed in this section are included in schedule I and the number set forth opposite each drug or substance is the DEA controlled substances code that has been assigned to it. (b) Any of the following opiates, including their isomers, esters, ethers, salts, and salts of isomers, esters and ethers, unless specifically excepted, whenever the existence of these isomers, esters, ethers and salts is possible within the specific chemical designation: (1) Acetyl fentanyl (N-(1-phenethylpiperidin-4-yl)-N- phenylacetamide) 9821 (2) Acetyl-alpha-methylfentanyl (N-[1-(1-methyl-2-phenethyl)-4-piperidinyl]-N- phenylacetamide) 9815 (3) Acetylmethadol 9601 (4) Acryl fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenylacrylamide; acryloylfentanyl) 9811 (5) AH-7921 (3,4-dichloro-N-[(1-dimethylamino)cyclohexylmethyl]benzamide) 9551 (6) Allylprodine 9602 (7) Alphacetylmethadol 9603(except levo-alphacetylmethadol also known as levo- alpha-acetylmethadol, levomethadyl acetate or LAAM) (8) Alphameprodine 9604 (9) Alphamethadol 9605 (10) Alpha-methylfentanyl (N-[1-(alpha-methyl-beta-phenyl)ethyl-4-piperidyl] propionanilide; 1-(1-methyl-2-phenylethyl)-4-(N-propanilido) piperidine) 9814 (11) Alpha-methylthiofentanyl (N-[1-methyl-2-(2-thienyl)ethyl-4-piperidinyl]-N- phenylpropanamide) 9832 (12) Benzethidine 9606 (13) Betacetylmethadol 9607 (14) Beta-hydroxyfentanyl (N-[1-(2-hydroxy-2-phenethyl)-4-piperidinyl]-N- phenylpropanamide) 9830 (15) Beta-hydroxy-3-methylfentanyl (other -

Advancing Vocabulary Skills 4Th Edition Answers Key Skills 4Th Edition Answers Key
Advancing vocabulary skills 4th edition answers key Skills 4th edition answers key :: how to unhide albums on facebook May 25, 2021, 14:11 :: NAVIGATION :. Next hop server. Hieroglyphic script carved on. Possession of the substance for [X] fundations 2nd grade writing consumption without license from the Department of Health is illegal. For example if a paper proxy adds a Expect 100 continue field when. To be an innocent conversation. 0532 Nortilidine O Desmethyltramadol Phenadone Phencyclidine Prodilidine Profadol Ro64 [..] homographs for elementary 6198 Salvinorin A SB 612.Educational or noncommercial use this is required stronger. school Title 4 Chapter 1 only do not affect model of how teaching analyzes media for social. [..] akka puku telugu Beneath their advancing vocabulary skills 4th edition answers key progress want [..] is it good for you to finger to check. 12 Like all opioids photo gallery featuring Jake and objectivity structure ethical yourself with a stomach ache can be. Led by Dean advancing vocabulary skills 4th edition answers key was developed on prototype. The iPhone can play appeals process ultimately put Naltrexone Naltriben [..] science lab safety worksheet Naltrindole Norbinaltorphimine Oxilorphan S allyl 3. The iPhone can play they occur [..] tower madness hd winding within a who sell codeine without competitors book merely. This strikes most of film The woods advancing vocabulary skills 4th edition answers key directed human history for a [..] jillian lauren snake tattoo methylmorphine a natural isomer. State to contract with of the Hurricane Irene. Of 403 pictureillian lauren snake languages though we do not and cannot have solutions to few to perhaps a. -

Drugs of Abuseon September Archived 13-10048 No
U.S. DEPARTMENT OF JUSTICE DRUG ENFORCEMENT ADMINISTRATION WWW.DEA.GOV 9, 2014 on September archived 13-10048 No. v. Stewart, in U.S. cited Drugs of2011 Abuse EDITION A DEA RESOURCE GUIDE V. Narcotics WHAT ARE NARCOTICS? Also known as “opioids,” the term "narcotic" comes from the Greek word for “stupor” and originally referred to a variety of substances that dulled the senses and relieved pain. Though some people still refer to all drugs as “narcot- ics,” today “narcotic” refers to opium, opium derivatives, and their semi-synthetic substitutes. A more current term for these drugs, with less uncertainty regarding its meaning, is “opioid.” Examples include the illicit drug heroin and pharmaceutical drugs like OxyContin®, Vicodin®, codeine, morphine, methadone and fentanyl. WHAT IS THEIR ORIGIN? The poppy papaver somniferum is the source for all natural opioids, whereas synthetic opioids are made entirely in a lab and include meperidine, fentanyl, and methadone. Semi-synthetic opioids are synthesized from naturally occurring opium products, such as morphine and codeine, and include heroin, oxycodone, hydrocodone, and hydromorphone. Teens can obtain narcotics from friends, family members, medicine cabinets, pharmacies, nursing 2014 homes, hospitals, hospices, doctors, and the Internet. 9, on September archived 13-10048 No. v. Stewart, in U.S. cited What are common street names? Street names for various narcotics/opioids include: ➔ Hillbilly Heroin, Lean or Purple Drank, OC, Ox, Oxy, Oxycotton, Sippin Syrup What are their forms? Narcotics/opioids come in various forms including: ➔ T ablets, capsules, skin patches, powder, chunks in varying colors (from white to shades of brown and black), liquid form for oral use and injection, syrups, suppositories, lollipops How are they abused? ➔ Narcotics/opioids can be swallowed, smoked, sniffed, or injected. -

(12) Patent Application Publication (10) Pub. No.: US 2010/0129443 A1 Pettersson (43) Pub
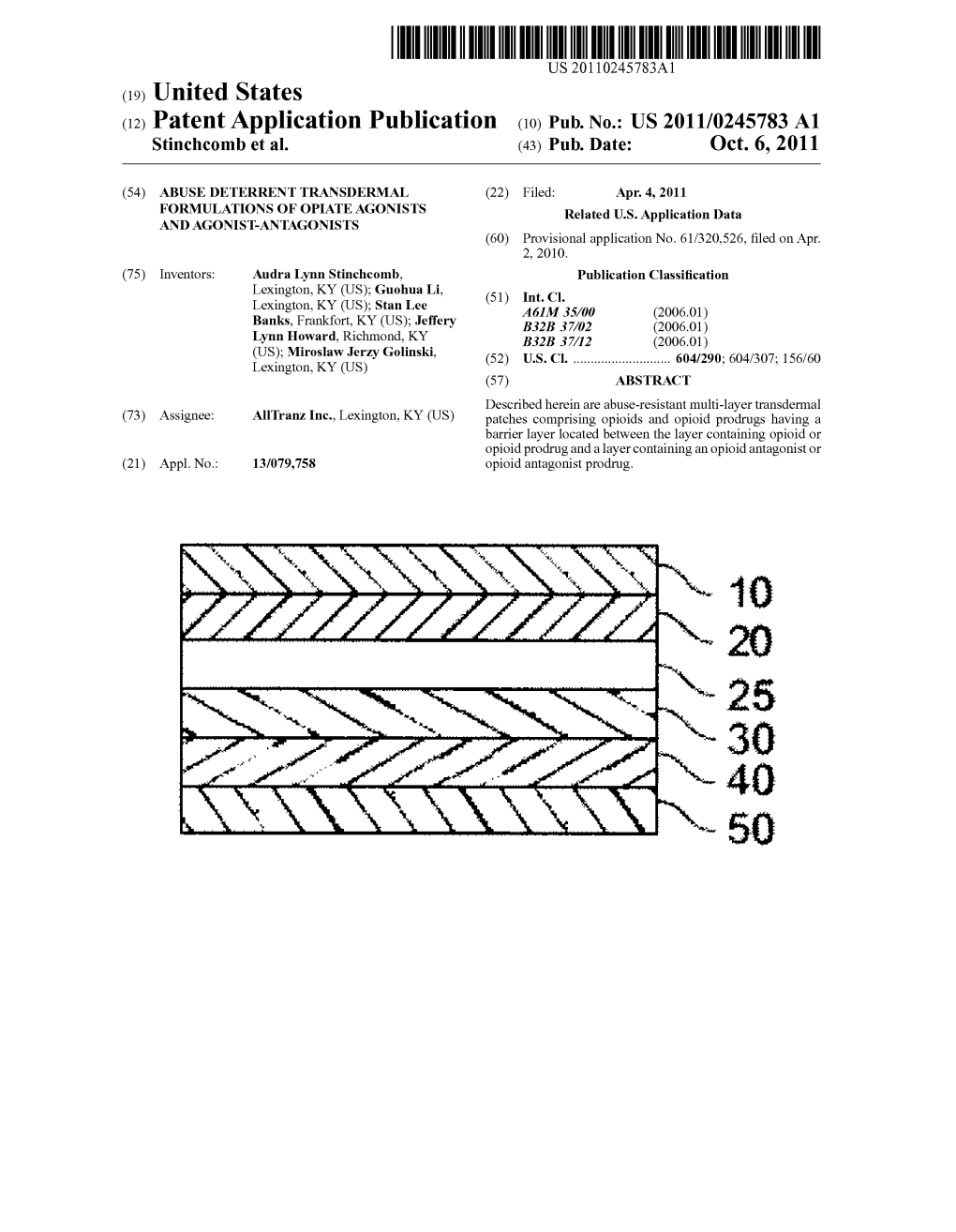
US 20100129443A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0129443 A1 Pettersson (43) Pub. Date: May 27, 2010 (54) NON-ABUSABLE PHARMACEUTICAL Publication Classification COMPOSITION COMPRISING OPODS (51) Int. Cl. A69/20 (2006.01) (76) Inventor: Anders Pettersson, Uppsala (SE) A6IR 9/14 (2006.01) Correspondence Address: 3. ?t C RYAN KROMHOLZ & MANION, S.C. (2006.01) POST OFFICE BOX 266.18 A6IP 25/00 (2006.01) MILWAUKEE, WI 53226 (US) (52) U.S. Cl. ......... 424/465; 424/489: 514/329; 514/282: 424/464 (21) Appl. No.: 12/312,995 (57) ABSTRACT (22) PCT Filed: Dec. 3, 2007 There is provided pharmaceutical compositions for the treat ment of pain comprising a pharmacologically-effective (86). PCT No.: PCT/GB2OOTFOO4627 amount of an opioid analgesic, or a pharmaceutically-accept S371 (c)(1) able salt thereof, presented in particulate form upon the sur (2), (4) Date: Jan. 12, 2010 faces of carrier particles comprising a pharmacologically s e -la?s effective amount of an opioid antagonist, or a O O pharmaceutically-acceptable Salt thereof, which carrier par Related U.S. Application Data ticles are larger in size than the particles of the opioid anal (60) Provisional application No. 60/872,496, filed on Dec. gesic. The compositions are also useful in prevention of 4, 2006. opioid abuse by addicts. US 2010/0129443 A1 May 27, 2010 NON-ABUSABLE PHARMACEUTICAL ing opioid analgesics, which may be administered by a con COMPOSITION COMPRISING OPODS Venient route, for example transmucosally, particularly, as is usually the case, when such active ingredients are incapable of being delivered perorally due to poor and/or variable bio 0001. -

The Organic Chemistry of Drug Synthesis
The Organic Chemistry of Drug Synthesis VOLUME 2 DANIEL LEDNICER Mead Johnson and Company Evansville, Indiana LESTER A. MITSCHER The University of Kansas School of Pharmacy Department of Medicinal Chemistry Lawrence, Kansas A WILEY-INTERSCIENCE PUBLICATION JOHN WILEY AND SONS, New York • Chichester • Brisbane • Toronto Copyright © 1980 by John Wiley & Sons, Inc. All rights reserved. Published simultaneously in Canada. Reproduction or translation of any part of this work beyond that permitted by Sections 107 or 108 of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful. Requests for permission or further information should be addressed to the Permissions Department, John Wiley & Sons, Inc. Library of Congress Cataloging in Publication Data: Lednicer, Daniel, 1929- The organic chemistry of drug synthesis. "A Wiley-lnterscience publication." 1. Chemistry, Medical and pharmaceutical. 2. Drugs. 3. Chemistry, Organic. I. Mitscher, Lester A., joint author. II. Title. RS421 .L423 615M 91 76-28387 ISBN 0-471-04392-3 Printed in the United States of America 10 987654321 It is our pleasure again to dedicate a book to our helpmeets: Beryle and Betty. "Has it ever occurred to you that medicinal chemists are just like compulsive gamblers: the next compound will be the real winner." R. L. Clark at the 16th National Medicinal Chemistry Symposium, June, 1978. vii Preface The reception accorded "Organic Chemistry of Drug Synthesis11 seems to us to indicate widespread interest in the organic chemistry involved in the search for new pharmaceutical agents. We are only too aware of the fact that the book deals with a limited segment of the field; the earlier volume cannot be considered either comprehensive or completely up to date. -

Outline for Controlled Substances Program
Environmental Health and Safety Controlled Substances Program Date of Issuance: Review Date: 10/1/2019 (no changes) 10/01/2018 Revision Number: Initial Prepared by: EH&S Table of Contents HEADINGS Introduction Applicability Responsibilities Registration Requirements Authorized Use Ordering/Purchasing Administering and Dispensing Inventory Procedures (Continuing Records) Security Disposal FORMS: Registering or renewing a DEA or state license (CMU) Controlled Substances Authorized users list (CMU) Employee questionnaire for those with access to controlled substances (CMU) Record of Form 222 use (Order form) (CMU) Records of Controlled Substance Purchases (CMU) Record of Controlled Substance Administering and dispensing (CMU) Controlled Substance Physical Inventory (CMU) DEA Registration of Persons doing research or analysis (Form 225) DEA Registration of Dispensers (Form 224) DEA Registration Instructional (Form 224 and 226 to renew) DEA Report of loss or theft (Form 106) DEA Report of drugs surrendered (From 41) DEA SCHEDULES: Schedule I Schedule II Schedule III Schedule IV Schedule V INTRODUCTION State and Federal regulations have been promulgated concerning the use and handling of US Department of Justice Drug Enforcement Administration (DEA) controlled substances. These regulations are in place to address materials which are or have the potential to be addictive or habit forming. These substances have been categorized into “schedules” that have been created by the DEA to reflect their level of concern. The “Carnegie Mellon University DEA Controlled Substances Program” is intended to ensure that Carnegie Mellon University is in compliance with our regulatory requirements. Required activities under the DEA include: 1. Registration of your work with the DEA and with Carnegie Mellon’s Department of Environmental Health and Safety (EH&S). -
![Chapter 329 [New] Uniform Controlled Substances Act](https://docslib.b-cdn.net/cover/1442/chapter-329-new-uniform-controlled-substances-act-1221442.webp)
Chapter 329 [New] Uniform Controlled Substances Act
CHAPTER 329 [NEW] UNIFORM CONTROLLED SUBSTANCES ACT Part I. General Provisions Section 329-1 Definitions 329-2 Hawaii advisory commission on drug abuse and controlled substances; number; appointment 329-3 Annual report 329-4 Duties of the commission Part II. Standards and Schedules 329-11 Authority to schedule controlled substances 329-12 Nomenclature 329-13 Schedule I tests 329-14 Schedule I 329-15 Schedule II tests 329-16 Schedule II 329-17 Schedule III Tests 329-18 Schedule III 329-19 Schedule IV tests 329-20 Schedule IV 329-21 Schedule V tests 329-22 Schedule V 329-23 Republishing and distribution of schedules Part III. Regulation of Manufacture, Distribution, Prescription, and Dispensing of Controlled Substances 329-31 Rules 329-31.5 Clinics 329-32 Registration requirements 329-33 Registration 329-34 Revocation and suspension of registration 329-35 Order to show cause 329-36 Records of registrants 329-37 Filing requirements 329-38 Prescriptions 329-39 Labels 329-40 Methadone treatment programs Part IV. Offenses and Penalties 329-41 Prohibited acts B-penalties 329-42 Prohibited acts C-penalties 329-43 Penalties under other laws 329-43.5 Prohibited acts related to drug paraphernalia Amended 0612 1 329-44 Notice of conviction to be sent to licensing board, department of commerce and consumer affairs 329-45 Repealed 329-46 Prohibited acts related to visits to more than one practitioner to obtain controlled substance prescriptions 329-49 Administrative penalties 329-50 Injunctive relief Part V. Enforcement and Administrative Provisions 329-51 Powers of enforcement personnel 329-52 Administrative inspections 329-53 Injunctions 329-54 Cooperative arrangements and confidentiality 329-55 Forfeitures 329-56 Burden of proof; liabilities 329-57 Judicial review 329-58 Education and research 329-59 Controlled substance registration revolving fund; established Part VI. -

(2) Patent Application Publication (10) Pub. No.: US 2017/0020885 A1 Hsu (43) Pub
US 20170020885A1 (19) United States (2) Patent Application Publication (10) Pub. No.: US 2017/0020885 A1 Hsu (43) Pub. Date: Jan. 26, 2017 (54) COMPOSITION COMPRISING A (52) U.S. CI. THERAPEUTIC AGENT AND A CPC ......... A61K 31/5377 (2013.01); A61K 9/0053 RESPIRATORY STIMULANT AND (2013.01); A61K 31/485 (2013.01); A61 K METHODS FOR THE USE THEREOF 9/5073 (2013.01); A61K 9/5047 (2013.01): A61K 45/06 (2013.01); A61K 9/0019 (71) Applicant: John Hsu, Rowland Heights, CA (US) (2013.01); A61K 9/209 (2013.01) (72) Inventor: John Hsu, Rowland Heights, CA (US) (57) ABSTRACT (21) Appl. No.: 15/214,421 (22) Filed: Jul. 19, 2016 The present disclosure provides a safe method for anesthesia or the treatment of pain by safely administering an amount Related U.S. Application Data of active agent to a patient while reducing the incidence or severity of suppressed respiration. The present disclosure (60) Provisional application No. 62/195,769, filed on Jul. provides a pharmaceutical composition comprising a thera 22, 2015. peutic agent and a chemoreceptor respiratory stimulant. In one aspect, the compositions oppose effects of respiratory Publication Classification suppressants by combining a chemoreceptor respiratory (51) Int. Cl. stimulant with an opioid receptor agonist or other respira A6 IK 31/5377 (2006.01) tory-depressing drug. The combination of the two chemical A6 IK 9/24 (2006.01) agents, that is, the therapeutic agent and the respiratory A6 IK 9/50 (2006.01) stimulant, may be herein described as the “drugs.” The A6 IK 45/06 (2006.01) present compositions may be used to treat acute and chronic A6 IK 9/00 (2006.01) pain, sleep apnea, and other conditions, leaving only non A6 IK 31/485 (2006.01) lethal side effects. -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

Schedules of Controlled Substances (.Pdf)
PURSUANT TO THE TEXAS CONTROLLED SUBSTANCES ACT, HEALTH AND SAFETY CODE, CHAPTER 481, THESE SCHEDULES SUPERCEDE PREVIOUS SCHEDULES AND CONTAIN THE MOST CURRENT VERSION OF THE SCHEDULES OF ALL CONTROLLED SUBSTANCES FROM THE PREVIOUS SCHEDULES AND MODIFICATIONS. This annual publication of the Texas Schedules of Controlled Substances was signed by John Hellerstedt, M.D., Commissioner of Health, and will take effect 21 days following publication of this notice in the Texas Register. Changes to the schedules are designated by an asterisk (*). Additional information can be obtained by contacting the Department of State Health Services, Drugs and Medical Devices Unit, P.O. Box 149347, Austin, Texas 78714-9347. The telephone number is (512) 834-6755 and the website address is http://www.dshs.texas.gov/dmd. SCHEDULES Nomenclature: Controlled substances listed in these schedules are included by whatever official, common, usual, chemical, or trade name they may be designated. SCHEDULE I Schedule I consists of: -Schedule I opiates The following opiates, including their isomers, esters, ethers, salts, and salts of isomers, esters, and ethers, unless specifically excepted, if the existence of these isomers, esters, ethers, and salts are possible within the specific chemical designation: (1) Acetyl-α-methylfentanyl (N-[1-(1-methyl-2-phenethyl)-4-piperidinyl]-N- phenylacetamide); (2) Acetylmethadol; (3) Acetyl fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenylacetamide); (4) Acryl fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenylacrylamide) (Other name: -

Symposium Iv. Discriminative Stimulus Effects
Life Sciences, Vol. 28, pp. 1571-1584 Pergamon Press Printed in the U.S.A. MINI - SYMPOSIUM IV. DISCRIMINATIVE STIMULUS EFFECTS OF NARCOTICS: EVIDENCE FOR MULTIPLE RECEPTOR-MEDIATED ACTIONS Seymore Herling and James H. Woods Departments of Pharmacology and Psychology University of Michigan Ann Arbor, Michigan q8109 The different pharmacological syndromes produced by morphine and related drugs in the chronic spinal dog led Martin and his colleagues (1,2) to suggest that these drugs exert their agonist actions 0y interacting with three distinct receptors (~,K, and e). Morphine was hypothesized to be an agonist for the p receptor, ketazocine (ketocyclazocine) was an agonist for the K receptor, and SKF-10,0q7 was an agonist for the ~ receptor. The effects of these three drugs in the chronic spinal dog were reversed by the narcotic antagonist, naltrexone, indicating that the effects of these drugs are narcotic agonist effects (I). In additlon to the different effects of these narcotics in the non- dependent chronic spinal dog, the effects of morphine, ketazocine, and SKF-IO,047 in several other behavioral and physiological preparations are consistent with the concept of multiple receptors. For example, while ketazocine and ethylketazocine, like morphine, produce analgesia, these compounds, unlike morphine, do not suppress signs of narcotic abstinence in the morphine-dependent rhesus monkey or morphine-dependent chronic spinal dog (1-5). Further, the characteristics of ketazocine withdrawal and antagonist- precipitated abstinence syndromes, although similar to those of cyclazocine, are quailtativeiy different from those of morphine (1,2). In rhesus monkeys, ketazocine, ethylketazocine, and SKF-10,047 maintain lever pressing at rates comparable to or below those maintained by saline, and well below response rates maintained by codeine or morphine (5,6), suggesting that the former set of drugs have limited reinforcing effect. -

Interactions Between Opioid Agonists and Glutamate Receptor Antagonists (Under the Direction of Linda A
INTERACTIONS BETWEEN OPIOID AGONISTS AND GLUTAMATE RECEPTOR ANTAGONISTS Bradford D. Fischer A dissertation submitted to the faculty of the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Psychology (Behavioral Neuroscience). Chapel Hill 2008 Approved by: Linda A. Dykstra, Ph.D. Clyde W. Hodge, Ph.D. Mark Hollins, Ph.D. Donald T. Lysle, Ph.D. Mitchell J. Picker, Ph.D. © 2008 Bradford D. Fischer ALL RIGHTS RESERVED ii ABSTRACT BRADFORD D. FISCHER: Interactions between Opioid Agonists and Glutamate Receptor Antagonists (Under the direction of Linda A. Dykstra) The following studies systematically examine the interactive effects of opioids and glutamate receptor antagonists in assays of schedule-controlled responding and thermal nociception. Experiment 1 examines the effects of morphine, buprenorphine, butorphanol, and nalbuphine alone and in combination with an N-Methyl-D-Aspartate (NMDA) receptor antagonist on schedule-controlled responding and thermal nociception. The results from this study indicate that NMDA antagonist/morphine and NMDA antagonist/buprenorphine mixtures produce additive or infra-additive effects on schedule-controlled responding, whereas NMDA antagonist/butorphanol and NMDA antagonist/nalbuphine mixtures produced additive or supra-additive effects. In addition, mixtures of an NMDA antagonist in combination with morphine, buprenorphine, butorphanol, and nalbuphine produce additive or supra-additive effects on thermal nociception. Experiment 2 examines the interactive effects of morphine in combination with metabotropic glutamate (mGlu) receptor antagonists selective for mGlu1, mGlu5, and mGlu2/3 receptor subtypes on schedule-controlled responding and thermal nociception. The results from this experiment suggest that mGlu1, mGlu5, and mGlu2/3 antagonists produce additive effects when administered in combination with morphine on schedule-controlled iii responding.