Leprosy: an Overview
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(ERYTHEMA NODOSUM LEPROSUM) the Term
? THE HISTOPATHOLOGY OF ACUTE PANNICULITIS NODOSA LEPROSA (ERYTHEMA NODOSUM LEPROSUM) w. J. PEPLER, M.B., Ch.B. Department of Pathology, South African Institute for Medical Research, Johannesburg R. KOOIJ, M.D. Westfort Institution, Pretoria AND J. MARSHALL, M.D. University of Pretoria, Pretoria The term "erythema nodosum leprosum" has frequently appeared in the literature on leprosy since the 1930's, apparently popularized by the Japanese (9). The occurrence of "erythema nodosum" in leprosy had previously been noted at the end of the nineteenth century by Brocq (6) and by Hansen and Looft (8), but the term seems to have fallen into disuse. As will become clear from our clinical and histological descriptions of the phenomenon commonly known as erythema nodosum leprosum, we believe the title to be a misnomer. We suggest that it would be more accurate to call it panniculitis nodosa leprosa (P.N.L,). This condition is commonly confused with acute lepra reaction, both processes being referred to as "acute reactions"; but a sharp line of distinction must be drawn between them. P.N.L. occurs only in patients with lepromatous leprosy as an acute, subacute or chronic skin eruption, with or without fever. It is characterized by showers of dusky red nodules, 0.5 to 2 cm. in diameter, on the extensor surfaces of the limbs, on the face, and, less often, on the trunk. The number of lesions appearing in an attack varies from a few to several hundreds, and they sometimes coalesce to form plaques. The nodules may be painful, and they are usually tender to touch. -

Chapter 3 Bacterial and Viral Infections
GBB03 10/4/06 12:20 PM Page 19 Chapter 3 Bacterial and viral infections A mighty creature is the germ gain entry into the skin via minor abrasions, or fis- Though smaller than the pachyderm sures between the toes associated with tinea pedis, His customary dwelling place and leg ulcers provide a portal of entry in many Is deep within the human race cases. A frequent predisposing factor is oedema of His childish pride he often pleases the legs, and cellulitis is a common condition in By giving people strange diseases elderly people, who often suffer from leg oedema Do you, my poppet, feel infirm? of cardiac, venous or lymphatic origin. You probably contain a germ The affected area becomes red, hot and swollen (Ogden Nash, The Germ) (Fig. 3.1), and blister formation and areas of skin necrosis may occur. The patient is pyrexial and feels unwell. Rigors may occur and, in elderly Bacterial infections people, a toxic confusional state. In presumed streptococcal cellulitis, penicillin is Streptococcal infection the treatment of choice, initially given as ben- zylpenicillin intravenously. If the leg is affected, Cellulitis bed rest is an important aspect of treatment. Where Cellulitis is a bacterial infection of subcutaneous there is extensive tissue necrosis, surgical debride- tissues that, in immunologically normal individu- ment may be necessary. als, is usually caused by Streptococcus pyogenes. A particularly severe, deep form of cellulitis, in- ‘Erysipelas’ is a term applied to superficial volving fascia and muscles, is known as ‘necrotiz- streptococcal cellulitis that has a well-demarcated ing fasciitis’. This disorder achieved notoriety a few edge. -

Leprosy – Eliminated and Forgotten: a Case Report Shiva Raj K.C.1,5* , Geetika K.C.1, Purnima Gyawali2, Manisha Singh3 and Milesh Jung Sijapati4
K.C. et al. Journal of Medical Case Reports (2019) 13:276 https://doi.org/10.1186/s13256-019-2198-1 CASE REPORT Open Access Leprosy – eliminated and forgotten: a case report Shiva Raj K.C.1,5* , Geetika K.C.1, Purnima Gyawali2, Manisha Singh3 and Milesh Jung Sijapati4 Abstract Background: Leprosy is a disease that was declared eliminated in 2010 from Nepal; however, new cases are diagnosed every year. The difficulty arises when the presentation of the patient is unusual. Case presentation: In this case report we present a case of a 22-year-old Tamang man, from the Terai region of Nepal, with a clinical presentation of fever, malaise, and arthralgia for the past 2 weeks with hepatosplenomegaly and bilateral cervical, axillary, and inguinal lymphadenopathy. Features of chronic inflammation with elevated erythrocyte sedimentation rate of 90 mm/hour and liver enzymes were noted. With no specific investigative findings, a diagnosis of Still’s disease was made and he was given prednisolone. On tapering the medication, after 2 weeks, the lymphadenopathy and fever reappeared. On biopsy of a lymph node, diagnosis of possible tuberculosis was made. On that basis anti-tuberculosis treatment category I was started. During his hospital stay, our patient developed nodular skin rashes on his shoulder, back, and face. The biopsy of a skin lesion showed erythema nodosum leprosum and he was diagnosed as having lepromatous leprosy with erythema nodosum leprosum; he was treated with anti-leprosy medication. Conclusion: An unusual presentations of leprosy may delay its prompt diagnosis and treatment; thus, increasing morbidity and mortality. -

Lepromatous Leprosy Simulating Sweet Syndrome
ISSN: 2469-5750 Zemmez et al. J Dermatol Res Ther 2018, 4:056 DOI: 10.23937/2469-5750/1510056 Volume 4 | Issue 1 Journal of Open Access Dermatology Research and Therapy CASE REPORT Lepromatous Leprosy Simulating Sweet Syndrome Youssef Zemmez1*, Ahmed Bouhamidi1, Salwa Belhabib2, Rachid Frikh1, Mohamed Boui1 1 and Naoufal Hjira Check for updates 1Department of Dermatology-Venereology, Mohammed V Military Training Hospital, Rabat, Morocco 2Department of Pathological Anatomy, Mohammed V Military Training Hospital, Rabat, Morocco *Corresponding author: Youssef Zemmez, Department of Dermatology-Venereology, Mohammed V Military Training Hospital, Rabat, Morocco, Tel: 0658150805, E-mail: [email protected] Abstract Leprosy or Hansen's disease is an infection by Mycobac- terium leprae (M. leprae), whose prevalence has consid- erably decreased since the application of the new anti-lep- rosy strategies advocated since 1982 by the World Health Organization (WHO). However, in the endemic countries several cases of leprosy are reported annually. We report a clinical case of lepromatous leprosy revealed by dissemi- nated maculopapular lesions simulating a Sweet syndrome highlighting the importance of knowing how to evoke this diagnosis in patients from endemic areas. Keywords Lepromatous leprosy, Rash, Sweet syndrome Introduction Lepromatous leprosy is generally manifested by Figure 1: a) Maculopapular erythematous lesions of the non-inflammatory lesions, hypochromic macules, and face; b) Papulonodular erythematous lesions in forearms progressive erythematous papulo-nodules. We report and hands. an observation of lepromatous leprosy in a 62-year-old patient from rural Morocco who was diagnosed with abdomen (Figure 2a and Figure 2b). The neurological maculopapular lesions. examination showed hypoesthesia in gloves and socks, with bilateral hypertrophy of the ulnar nerve. -
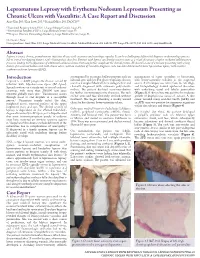
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting As
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting as Chronic Ulcers with Vasculitis: A Case Report and Discussion Anny Xiao, DO,* Erin Lowe, DO,** Richard Miller, DO, FAOCD*** *Traditional Rotating Intern, PGY-1, Largo Medical Center, Largo, FL **Dermatology Resident, PGY-2, Largo Medical Center, Largo, FL ***Program Director, Dermatology Residency, Largo Medical Center, Largo, FL Disclosures: None Correspondence: Anny Xiao, DO; Largo Medical Center, Graduate Medical Education, 201 14th St. SW, Largo, FL 33770; 510-684-4190; [email protected] Abstract Leprosy is a rare, chronic, granulomatous infectious disease with cutaneous and neurologic sequelae. It can be a challenging differential diagnosis in dermatology practice due to several overlapping features with rheumatologic disorders. Patients with leprosy can develop reactive states as a result of immune complex-mediated inflammatory processes, leading to the appearance of additional cutaneous lesions that may further complicate the clinical picture. We describe a case of a woman presenting with a long history of a recurrent bullous rash with chronic ulcers, with an evolution of vasculitic diagnoses, who was later determined to have lepromatous leprosy with reactive erythema nodosum leprosum (ENL). Introduction accompanied by an intense bullous purpuric rash on management of sepsis secondary to bacteremia, Leprosy is a slowly progressive disease caused by bilateral arms and face. For these complaints she was with lower-extremity cellulitis as the suspected infection with Mycobacterium leprae (M. leprae). seen in a Complex Medical Dermatology Clinic and source. A skin biopsy was taken from the left thigh, Spread continues at a steady rate in several endemic clinically diagnosed with cutaneous polyarteritis and histopathology showed epidermal ulceration countries, with more than 200,000 new cases nodosa. -

Leprosy' Revi"Ew
LEPROSY' REVI"EW lbe QD8rterIy Publication of THE BRITISH LEPROSY RELIEF ASSOCIAll0N VOL. XXVIII. No 3. JULY 1957 Principal Contents EditoriaIs Secondary Infections and Neoplasms in Leprosy Patients. Leprosy of the Eye. Thiosemicarbazone in the Treatment of the Reactional and Bordeline Forms of Leprosy. Some Data on the Influence of B.C.G. Vaccination in Leprosy Patients. Abstracts 8 PORTMAN STREET, LONDON, W.l Price: Three Shillings and Sixpence, plus posfage Annual Subscripfion: Fiffeen Shillings, including posfage LEPROSY REVIEW VOL. XXVIII, No. 3. JULY, 1957 CONTENTS PAGE Editorials: Corticosteroids in Leprosy 91 Is there a place for hypnotherapy in leprosy treatment? 92 Secondary Infections and Neoplasms in Leprosy Patients FELIX CONTRERAS 95 Leprosy of the Eye .. W. ]. HOLMES 108 Thiosemicarbazone in the Treatment of the Reactional and Borderline Forms of Leprosy H. W. WHEATE 124 Some Data on the Influence of BCG Vaccination in Leprosy Patients ]. VAN DE HEYNING 130 Abstracts 131 Edited by DR. J. Ross INNES, Medical Secretary of the British Leprosy Relief Association, 8 Portman Street, London, W.l, to whom all communications should be sent. The Association does not accept responsibilty for views expressed by writers. Contributors of original articles will receive 25 loose reprints free, but more formal bound reprints must be ordered at time of submitting the article, and the cost reimbursed later. THE ONE DOSE REMEDY , ANTEPAR ' is such a simple and practical ascarifuge, it enables you to tackle tbe roundworm problem on a large scale. You simply givc each adult or child onc dosc of 'A TEPAR' }!'Iixir. 0 purging, fasting or dieling is necessary. -

Lepromatous Leprosy Masquerading As Rhinophyma
International Journal of Otorhinolaryngology and Head and Neck Surgery Krishna S et al. Int J Otorhinolaryngol Head Neck Surg. 2015 Jul;1(1):34-36 http://www.ijorl.com pISSN 2454-5929 | eISSN 2454-5937 DOI: http://dx.doi.org/10.18203/issn.2454-5929.ijohns20150585 Case Report Lepromatous leprosy masquerading as rhinophyma 1 1 1 2 Sowmyashree Krishna *, Malcolm Pinto , Manjunath Mala Shenoy , Mahesh SG 1 Department of Dermatology, Yenepoya Medical College, Yenepoya University, Mangalore, Karnataka, India 2Department of Otolaryngology-Head and Neck Surgery, A.J. Institute of Medical Sciences and Research Center, Mangalore, Karnataka, India Received: 26 May 2015 Accepted: 24 June 2015 *Correspondence: Dr. Sowmyashree Krishna, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Leprosy a major global health problem, especially in the developing world, is an infectious disease caused by Mycobacterium leprae. Leprosy has a predilection to with cooler areas of the body. Lepromatous leprosy presents with varied manifestations like nodules, cervical lymphadenitis, hyperpigmented patches and other presentations which can mimic various other diseases and pose a diagnostic challenge in endemic areas. We report a case presenting with nodular infiltration of the nose mimicking rhinophyma who presented with faint reddish swelling over the nose which progressed to nodular infiltration. There was bilateral symmetrical thickening of nerves following which diagnosis was confirmed by slit skin smear and the patient was started on multibacillary multidrug therapy. -

A Clinicopathological Analysis of Granulomatous
JK SCIENCE ORIGINAL ARTICLE A Clinicopathological Analysis of Granulomatous Dermatitis : 4 Year Retrospective Study Jyotsna Suri, Subhash Bhardwaj, Rita Kumari, Shailija Kotwal Abstract The present study was carried out with an attempt to study the incidence of granulomatous dermatitis in hospital based population and to classify and compare the granulomatous dermatitis on the basis of histopathology and find the etiology. This is a four year retrospective study done on the data available in the dermatopathology section of department of pathology. The cases diagnosed as granulomatous dermatitis were retrieved, clinical data and the histopathological features compared to know the incidence of various etiologies of GD. Out of 310 cases of GD with male to female ratio 2.03:1, leprosy comprised major reported etiology (n-244) followed by tuberculosis (n-44), sarcoidosis (n-4), Leshmania Donovani (n-14)) granuloma annulare (n-1) and 3 granulomatous lesions not further classified. Infections form the commonest form of GD out of which leprosy forms the major group. Role of histopathology(H&E and special stains) is very important in confirming the diagnosis of granulomatous dermatitis . Key Words Granuloma, Dermatitis, Inflammatory, Tissue Injury Introduction Granuloma is defined as a focal chronic inflammatory dermatitis presents a diagnostic challenge, many a times. response to tissue injury, characterised by focal, compact The granulomatous dermatitis comprise a large family collection of inflammatory cells, principally of the activated sharing the common histological denominator of histiocytes, modified epitheloid macrophages & granulomas formation. Rightly said that in granulomatous multinucleate giant cells that may or may not be rimmed dermatitis, an identical histologic picture may be produced by lymphocytes and show central necrosis. -

Histopathological Study of Dermal Granuloma Gunvanti B
https://ijmsweb.com Indian Journal of Medical Sciences Original Article Histopathological study of dermal granuloma Gunvanti B. Rathod1, Pragnesh Parmar2 1Departments of Pathology, 2Forensic Medicine, GMERS Medical College, Vadnagar, Gujarat, India. ABSTRACT Introduction: The objectives of this study were to confirm the diagnosis of clinically suspected dermal granuloma- tous diseases by histopathological examination and by routine and special stains as well as to study the incidence of various types of dermal granulomas. *Corresponding author: Materials And Methods: This study was conducted at the Department of Pathology in collaboration with De- partment of Skin and Venereal disease. A total of 90 cases from outdoor patient department of skin and venereal Dr. Gunvanti Rathod, disease, which were clinically diagnosed as suspected dermal granulomatous diseases, were taken as the study Departments of Pathology, population. GMERS Medical College, Vadnagar, Gujarat, India. Results: In our study, we found that leprosy had the highest incidence (50%), followed by cutaneous tuberculosis (30%) among all dermal granulomatous diseases like syphilis, fungal, granuloma annulare, foreign body, actino- [email protected] mycosis, and sarcoidosis. Dermal granulomas were most common in middle age between 21 and 40 years of age. Received : 18 September 19 Conclusion: Histopathology played an important role in the final diagnosis of dermal granulomatous lesions. Most common dermal granulomatous disease was leprosy, followed by cutaneous tuberculosis. -

6/12/2018 1 Infectious Dermatopathology
6/12/2018 Infectious Dermatopathology – What is “bugging” you? Alina G. Bridges, D.O. Associate Professor Program Director, Dermatopathology Fellowship ASDP Alternate Advisor to the AMA-RUC Department of Dermatology, Division of Dermatopathology and Cutaneous Immunopathology Mayo Clinic, Rochester Disclosures ▪Relevant Financial Relationships ▪None ▪Off Label Usage ▪No Background ▪ One of the most challenging tasks in medicine is the accurate and timely diagnosis of infectious diseases ▪ Classically, infectious disease diagnosis is under the domain of the microbiology laboratory ▪ Culture ▪ May not be performed due to lack of clinical suspicion ▪ Time consuming ▪ Not possible for some organisms ▪ May fail to grow organism due to prior antibiotic treatment, sampling error 1 6/12/2018 Background, continued ▪ Infectious diseases has always played a significant role in our specialty ▪ Many infectious diseases have primary skin manifestations ▪ Dermatopathologists offer invaluable information: ▪ Knowledge of inflammatory patterns and other similar appearing non-infectious conditions ▪ Visualize microorganisms and associated cellular background ▪ Colonization versus Invasion Course Objectives At the end of this course, participants should be able to: ▪ Identify common and important infectious organisms in dermatopathology specimens based on histopathologic features ▪ Develop an appropriate differential diagnosis based on morphologic features, clinical presentation, exposure history, and corresponding microbiology results ▪ Use an algorithm -

INTERNATIONAL JOURNAL of LEPROSY Volume 72, Number 1 Printed in the U.S.A
INTERNATIONAL JOURNAL OF LEPROSY Volume 72, Number 1 Printed in the U.S.A. (ISSN 0148-916X) INTERNATIONAL JOURNAL OF LEPROSY and Other Mycobacterial Diseases VOLUME 72, NUMBER 1MARCH 2004 Relapses in Multibacillary Patients Treated with Multi-drug Therapy until Smear Negativity: Findings after Twenty Years1 Gift Norman, Geetha Joseph, and Joseph Richard2 ABSTRACT The Schieffelin Leprosy Research and Training Center at Karigiri, India participated in several of the World Health Organization (WHO) trials. The first trial on combined therapy in multi-bacillary leprosy was initiated in 1981. The main objectives of this field trial were to evaluate the efficacy of WHO recommended regimens in preventing relapses, especially drug resistance relapses. This paper reports on the relapses twenty years after patients were inducted into the WHO field trial. Between 1981 and 1982, 1067 borderline lepromatous and lepromatous patients were in- ducted into the WHO field trial for combined therapy in multi-bacillary leprosy trial. Among them, 357 patients were skin smear positive. During the follow-up in 2002, only 173 of them could be traced and assessed. The mean duration of follow-up was 16.4 ± 1.83 years. Two patients relapsed 14 and 15 years after being released from treatment, the relapse rate being 0.07 per 100 person years follow-up. Drug susceptibility tests done on one of the relapsed patients revealed drug sensitive organisms to all multi-drug therapy drugs. RÉSUMÉ Le centre de recherche et de formation de Schieffelin à Karigiri aux Indes a participé à plusieurs études cliniques sponsorisées par l’Organisation Mondiale de la Santé (OMS). -

Cutaneous Neurofibromas: Clinical Definitions Current Treatment Is Limited to Surgical Removal Or Physical Or Descriptors Destruction
ARTICLE OPEN ACCESS Cutaneous neurofibromas Current clinical and pathologic issues Nicolas Ortonne, MD, PhD,* Pierre Wolkenstein, MD, PhD,* Jaishri O. Blakeley, MD, Bruce Korf, MD, PhD, Correspondence Scott R. Plotkin, MD, PhD, Vincent M. Riccardi, MD, MBA, Douglas C. Miller, MD, PhD, Susan Huson, MD, Dr. Wolkenstein Juha Peltonen, MD, PhD, Andrew Rosenberg, MD, Steven L. Carroll, MD, PhD, Sharad K. Verma, PhD, [email protected] Victor Mautner, MD, Meena Upadhyaya, PhD, and Anat Stemmer-Rachamimov, MD Neurology® 2018;91 (Suppl 1):S5-S13. doi:10.1212/WNL.0000000000005792 Abstract RELATED ARTICLES Objective Creating a comprehensive To present the current terminology and natural history of neurofibromatosis 1 (NF1) cuta- research strategy for neous neurofibromas (cNF). cutaneous neurofibromas Page S1 Methods NF1 experts from various research and clinical backgrounds reviewed the terms currently in use The biology of cutaneous fi for cNF as well as the clinical, histologic, and radiographic features of these tumors using neuro bromas: Consensus published and unpublished data. recommendations for setting research priorities Results Page S14 Neurofibromas develop within nerves, soft tissue, and skin. The primary distinction between fi fi Considerations for cNF and other neuro bromas is that cNF are limited to the skin whereas other neuro bromas development of therapies may involve the skin, but are not limited to the skin. There are important cellular, molecular, for cutaneous histologic, and clinical features of cNF. Each of these factors is discussed in consideration of neurofibroma a clinicopathologic framework for cNF. Page S21 Conclusion Clinical trial design for The development of effective therapies for cNF requires formulation of diagnostic criteria that cutaneous neurofibromas encompass the clinical and histologic features of these tumors.