Valued–Priced Medication List (Cont.) 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Allergy Testing and History Form
Skin Testing Information and Consent 1. Skin Testing An allergy skin test is used to identify the substances that are causing your allergy symptoms. We will apply several extracts of common allergens to the skin and observe for a reaction. The reactions are then graded and confirmatory intradermal testing may be performed. This involves placing a small amount of extract under the skin of the upper arm. We then observe the reaction and record the results. On the day of testing please wear a short-sleeved shirt that can be pushed up comfortably to your shoulder. Allow 1-2 hours for your test session. You will need to stay on the premises during this time. Please do not bring children to your appointment. 2. Risks of Skin Testing Bleeding and infection may occur due to abrading of the skin. Any time the skin integrity is broken it puts on at risk for infection. However, this is a rare occurrence. The antigens used for testing are sterile and approved by the FDA. Occasionally, skin testing can trigger a severe allergic reaction requiring treatment with medications and/or treatment in the ER. Patients with asthma are at increased risk for triggering an asthma attack during testing. You should not undergo testing if you feel that you have allergy or asthma symptoms are currently under poor control. 3. Contraindications to Skin Testing Women who are pregnant or anyone who is currently taking Beta-Blockers, Tricyclic Anti-depressants or MAOI’s medications will NOT be skin tested. Please be sure to inform us of ALL your medications before the skin test is applied. -

Supporting Information a Analysed Substances
Electronic Supplementary Material (ESI) for Analyst. This journal is © The Royal Society of Chemistry 2020 List of contents: Tab. A1 Detailed list and classification of analysed substances. Tab. A2 List of selected MS/MS parameters for the analytes. Tab. A1 Detailed list and classification of analysed substances. drug of therapeutic doping agent analytical standard substance abuse drug (WADA class)* supplier (+\-)-amphetamine ✓ ✓ S6 stimulants LGC (+\-)-methamphetamine ✓ S6 stimulants LGC (+\-)-3,4-methylenedioxymethamphetamine (MDMA) ✓ S6 stimulants LGC methylhexanamine (4-methylhexan-2-amine, DMAA) S6 stimulants Sigma cocaine ✓ ✓ S6 stimulants LGC methylphenidate ✓ ✓ S6 stimulants LGC nikethamide (N,N-diethylnicotinamide) ✓ S6 stimulants Aldrich strychnine S6 stimulants Sigma (-)-Δ9-tetrahydrocannabinol (THC) ✓ ✓ S8 cannabinoids LGC (-)-11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THC-COOH) S8 cannabinoids LGC morphine ✓ ✓ S7 narcotics LGC heroin (diacetylmorphine) ✓ ✓ S7 narcotics LGC hydrocodone ✓ ✓ Cerillant® oxycodone ✓ ✓ S7 narcotics LGC (+\-)-methadone ✓ ✓ S7 narcotics Cerillant® buprenorphine ✓ ✓ S7 narcotics Cerillant® fentanyl ✓ ✓ S7 narcotics LGC ketamine ✓ ✓ LGC phencyclidine (PCP) ✓ S0 non-approved substances LGC lysergic acid diethylamide (LSD) ✓ S0 non-approved substances LGC psilocybin ✓ S0 non-approved substances Cerillant® alprazolam ✓ ✓ LGC clonazepam ✓ ✓ Cerillant® flunitrazepam ✓ ✓ LGC zolpidem ✓ ✓ LGC VETRANAL™ boldenone (Δ1-testosterone / 1-dehydrotestosterone) ✓ S1 anabolic agents (Sigma-Aldrich) -
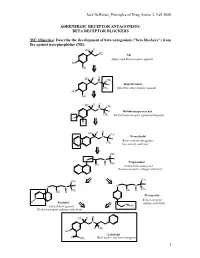
BETA RECEPTOR BLOCKERS MC Objective
Jack DeRuiter, Principles of Drug Action 2, Fall 2000 ADRENERGIC RECEPTOR ANTAGONISTS: BETA RECEPTOR BLOCKERS MC Objective: Describe the development of beta antagonists ("beta blockers") from the agonist norepinephrine (NE): HO H NH2 NE Alpha- and Beta-receptor agonist HO OH H H HO CH N 3 H Isoproterenol CH3 Selective beta-receptor agonist HO OH H H HO CH N 3 H Dichloroisoproterenol CH3 Partial beta-receptor agonist/antagonist Cl Cl H H HO CH N 3 Pronethalol H Beta-receptor antagonist, CH 3 low activity and toxic CH3 O N H Propranolol CH3 OH H Potent beta-antagonist No beta-receptor subtype selectivity CH3 CH3 O N H O N H CH CH OH H 3 OH H 3 Metoprolol N Beta-1-receptor H Pindolol O subtype selectivity Partial beta-agonist CH3 No beta-receptor subtype selectivity HO H H N H CH3 HO Labetolol O NH2 Dual alpha- and beta-antagonst 1 Jack DeRuiter, Principles of Drug Action 2, Fall 2000 MC Objective: Based on their structures, would the beta-blockers be expected to be relatively receptor selective? YES. They do not produce significant blockade of alpha- adrenergic receptors (alpha-1 or alpha-2), histamine receptors, muscarinic receptors or dopamine receptors. MC/PC Objective: Identify which beta blockers are classified as "non-selective": · The “non-selective" classification refers to those beta-blockers capable of blocking BOTH beta-1 and beta-2 receptors with equivalent efficacy. These drugs DO NOT have clinically significant affinity for other neurotransmitter receptors (alpha, dopamine, histamine, acetylcholine, etc.). · ALL of these beta-blockers (except satolol) consist of an aryloxypropanolamine side chain linked to an aromatic or “heteroaromatic” ring which is “ortho” substituted. -

CARTEOLOL HYDROCHLORIDE- Carteolol Hydrochloride Solution Sandoz Inc ------Carteolol Hydrochloride Ophthalmic Solution USP, 1% Rx Only Sterile
CARTEOLOL HYDROCHLORIDE- carteolol hydrochloride solution Sandoz Inc ---------- Carteolol Hydrochloride Ophthalmic Solution USP, 1% Rx Only Sterile DESCRIPTION Carteolol Hydrochloride Ophthalmic Solution USP, 1% is a nonselective beta-adrenoceptor blocking agent for ophthalmic use. The chemical name for carteolol hydrochloride is (±)-5-[3-[(1,1-dimethylethyl) amino]-2- hydroxypropoxy]-3,4-dihydro-2(1H)-quinolinone monohydrochloride. The structural formula is as follows: C16H24N2O3•HCI Mol. Wt. 328.84 Each mL of sterile solution contains Active: carteolol hydrochloride 10 mg (1%). Preservative: benzalkonium chloride 0.05 mg (0.005%). Inactives: sodium chloride, monobasic and dibasic sodium phosphate, sodium hydroxide and/or hydrochloric acid (to adjust pH to 6.0 - 8.0) and purified water. CLINICAL PHARMACOLOGY Carteolol is a nonselective beta-adrenergic blocking agent with associated intrinsic sympathomimetic activity and without significant membrane-stabilizing activity. Carteolol Hydrochloride reduces normal and elevated intraocular pressure (IOP) whether or not accompanied by glaucoma. The exact mechanism of the ocular hypotensive effect of beta-blockers has not been definitely demonstrated. In general, beta-adrenergic blockers reduce cardiac output in patients in good and poor cardiovascular health. In patients with severe impairment of myocardial function, beta-blockers may inhibit the sympathetic stimulation necessary to maintain adequate cardiac function. Beta-adrenergic blockers may also increase airway resistance in the bronchi and bronchioles due to unopposed parasympathetic activity. Given topically twice daily in controlled domestic clinical trials ranging from 1.5 to 3 months, Carteolol Hydrochloride produced a median percent reduction of IOP 22% to 25%. No significant effects were noted on corneal sensitivity, tear secretion, or pupil size. -

San Diego ENT
San Diego ENT Allergy Skin Testing Instructions Make sure you review all of your medications with your doctor or the medical assistant when you are scheduled for your allergy test. DON’T’S: • Do note take over-the-counter antihistamines, cold medication, or cough syrup for 10 days prior to the test. This includes Benadryl, Claritin, Zyrtec, Allegra, loratadine, cetirizine, and fexofenadine, Tavist, Dramamine,Atarax, and others. • Do not take prescription antihistamines for 10 days prior to the test including Claritin, Zyrtec, Allegra, loratadine, cetirizine, fexofenadine, and Astelin nasal spray. Also stop antihistamine eye drops 10 days prior to testing. • Do not take beta blockers 5 days prior to testing. These include labetalol, metoprolol, carvedilol, and their brand name equivalents. • Do not take anti-acid medication for 48 hours prior to testing including Zantac, Pepcid, and Tagamet. You may continue to take proton pump inhibitors such as Prilosec, Nexium, Prevacid, Protonix, and Aciphex. • Do not take any sleeping medications for 48 hours prior to testing including Tylenol PM and Excedrin PM. DO’S: • Wear something comfortable that will allow access to your back or both upper arms on the day of testing. • You may continue to use nasal steroid sprays such as Flonase, Nasonex, Nasacort, and Rhinocort. Do not use Astelin. • Review the list of medications that need to be avoided below. Antihistamines to stop 10 days prior to testing: Generic Brand Name Acrivastine Semprex Azatadine Optimine, Trinalin Bropheniramine AccuHist, Bromfed, -

Draft Guidance on Brimonidine Tartrate
Contains Nonbinding Recommendations Draft Guidance on Brimonidine Tartrate This draft guidance, when finalized, will represent the current thinking of the Food and Drug Administration (FDA, or the Agency) on this topic. It does not establish any rights for any person and is not binding on FDA or the public. You can use an alternative approach if it satisfies the requirements of the applicable statutes and regulations. To discuss an alternative approach, contact the Office of Generic Drugs. Active Ingredient: Brimonidine tartrate Dosage Form; Route: Solution/drops; ophthalmic Strength: 0.15% Recommended Studies: Two options: waiver or in vivo study Additional comments: Brimonidine tartrate ophthalmic solution products should have comparable physicochemical properties to the Reference Standard (RS) including but not limited to pH, specific gravity, buffer capacity, osmolality, and viscosity, if applicable. Comparative analysis should be performed on three exhibit batches, if available, of both test and RS products. I. Waiver option: To qualify for a waiver of the in vivo bioequivalence (BE) study requirement, a generic brimonidine tartrate ophthalmic solution product must be qualitatively (Q1)1 and quantitatively (Q2)2 the same as the Reference Listed Drug (RLD). An in vivo BE study with clinical endpoints is requested for any brimonidine tartrate ophthalmic solution product that has a different inactive ingredient from the RLD,3 a difference of more than 5% in the amount of any inactive ingredient compared to that of the RLD, or differences in comparative physicochemical characterization data. II. In Vivo option: Recommended studies: One study Type of study: BE study with clinical endpoint 1 Q1 (Qualitative sameness) means that the test product uses the same inactive ingredient(s) as the reference product. -

Medicines That Adversely Affect Allergy Skin Testing
1 OSU Department of Otolaryngology–Head and Neck Surgery Division of Sinus and Allergy at the OSU Eye and Ear Institute 915 Olentangy River Rd. 4th Floor Columbus, Oh 43212 614-366-ENTS (3687) Medicines that affect allergy skin testing: Do not take these medications 5 days or longer (as listed below), prior to your appointment: *This is not an all-inclusive list, review all the ingredients on the package or ask your doctor or pharmacist.* Antihistamines - First Generation Generic Name Brand Names Azatadine Optimine Bromphenarimine BroveX, Dimetane, Lodrane Carbinoxamine Maleate Histex Pd, Palgic and Pediatex AHIST, Aller-Chlor, C.P.M, Chlo-Amine, Chlor-Allergy, Chlor-Mal, ChlorTrimeton, Chlorphen, Chlorpheniramine (6 days) Effidac-24, Histex, Ridraman Clemastine (10 days) Allerhist-1, Contact 12hr Allergy, Tavist-1 Cyproheptadine (11 days) Periactin Dexchlorpheniramine Polaramine Actifed Sinus Day, Aler-Tab, Allergy, AllergySinus, Allermax, Aler-Dryl, Altaryl, Banophren, Benadryl, Calm-Aid, Children's Allergy, Compoz Nighttime, Diphedryl, Diphen-Allergy, Diphenhist, Dormin Sleep Aide, Dytan, Dytuss, Genahist, Hydramine, Ibuprofen PM, Nu-Med, Nytol, PediaCare Diphenhydramine Children's Allergy, Q-Dryl, Quenalin, Scot-Tussin Allergy, Siladryl, Silphen, Simply Allergy, Simply Sleep, Sleep-ettes, Sleep Formula, Sleepinal, Sominex, Tavist, Theraflu, Triaminic, Twilite, Tylenol PM, Unisom Sleep Gels, Valu-Dryl Dimenhydrinate Dramamine Hydroxyzine (8 days) Atarax, Rezine, Vistaril Ketotifen Zatiden Meclizine HCl Antivert, Bonine Methdilazine -

These Medications Should Not Be Taken at Least 14 Days Before Skin Testing**
**THESE MEDICATIONS SHOULD NOT BE TAKEN AT LEAST 14 DAYS BEFORE SKIN TESTING** Prescription and Over the Counter Nasal Sprays Antihistamines Accuhist (brompheniramine) Astelin (azelastine) Actifed (chlorpheniramine) Patanase (olopatadine) Advil Allergy Sinus Astepro (azelastine) Alavert (loratadine) Dymista Allegra/Allegra D (fexofenadine) AlleRx (chlorpheniramine Eye Drops Atarax/Vistaril (hydroxyzine) Bepreve (bepotastine) Benadryl (diphenhydramine) Elestat (epinastine) Biohist LA (chlorpheniramine) Naphcon-A (pheniramine) Bromfed/Bromfed PD (brompheniramine) Optivar (azelastine) Chlor-Trimeton (chlorpheniramine) Pataday (olopatadine) Claritin/Claritin D (loratadine) Patanol (olopatadine) Clarinex/Clarinex D (desloratadine) Visine-A (pheniramine) Deconamine/Naldecon (chlorpheniramine) Zaditor (Ketotifen) Dimetane (brompheniramine) Alaway Dimetapp (brompheniramine) Donatussin (carbinoxamine) Non-Prescription Sleep Aids Histex (brompheniramine) Advil PM (diphenhydramine) Histussin D/Histussin HC (Dexbrompheniramine) Nytol (diphenhydramine) Nalex-A (chlorpheniramine, phenyltoloxamine) Tylenol PM (diphenhydramine) Nyquil (doxylamine) Unisom (doxylamine) Palgic/Palgic-D (carbinoxamine) Pancof HC (chlorpheniramine) Antacid Medications Pannaz (carbinoxamine, methscopolamine) Axid (nizatidine) Periactin (cyproheptadine) Pepcid (famotidine) Polaramine (dexchlorpheniramine) Tagment (cimetadine) Poly-Histine (pheniramine, pyrilamine, Zantac (ranitidine) phenyltoloxamine) Rescon (chlorpheniramine) Respa-AR (chlorpheniramine) Anti-nausea/Anti-vertigo -

Ocular Toxicology and Pharmacology
Ocular Toxicology and Pharmacology Susan Schneider, MD Vice President, Retina Acucela Inc [email protected] Ocular Toxicology “The remedy often times proves worse than the disease” - William Penn Ocular Toxicology Site Affected Ocular Toxicology: Corneal and Lenticular • Chloroquine & hydroxychloroquine • Indomethacin • Amiodarone • Tamoxifen • Suramin • Chlorpromazine (corneal endothelium) • Gold salts (chrysiasis) Causes of Corneal “Swirl” Keratopathy • Chloroquine • Suramin (used in AIDS patients) • Tamoxifen • Amiodarone Ocular Toxicology: Transient Myopia • Sulfonamides • Tetracycline • Perchlorperazine (Compazine) • Steroids • Carbonic anhydrase inhibitors Ocular Toxicology: Conjunctival, Eyelid, Scleral • Isoretinoin: DES, blepharoconjunctivitis • Chlorpromazine: Slate-blue discoloration • Niacin: Lid edema • Gold salts: Conjunctiva • Tetracycline: Conjunctival inclusion cysts • Minocycline: Bluish discoloration of sclera Ocular Toxicology: Uveal Rifabutin: • Anterior uveitis +/- vitritis, associated with hypopyon • Resolves after discontinuation of medication Ocular Toxicology: Lacrimal system Decreased tearing: Increased tearing: • Anticholinergics • Adrenergic agonists • Antihistamines • Antihypertensives • Vitamin A analogs • Cholinergic agonists • Phenothiazines • Antianxiety agents • Tricyclic antidepressants Ocular Toxicology: Retinal • Chloroquine (Aralen) & Hydroxychloroquine (Plaquenil): Bull’s eye maculopathy • Thioridazine (Mellaril): +/-decreased central vision, pigment stippling, circumscribed RPE dropout • Quinine: -

ANTIHISTAMINES Antihistamines Need to Be Stopped 7 Days Prior to Allergy Testing
ANTIHISTAMINES Antihistamines need to be stopped 7 days prior to allergy testing Actifed Cetirizine Extendryl Rutuss Advil Allergy Chlorphenirmine Fexofenadine Ryna Advil PM Clortrimeton Hydroxyzine Rynatan Alavert Clarinex Hyzine Ryneze Allegra Claritin Lodrane Semprex Allerhist Clemastine Loratadine Singlet Allertan Cogentin Nyquil Sominex Allerx Comtrex Omnaris nasal spray Astelin nasal spray Contac Optivar eye drops Tandur Astepro nasal spray Coricidin Pataday Travist Atarax Cyproheptadine Patanase nasal spray Temaril Atrohist Deconamine Patanol eye drops Theraflu BC Cold Dimetapp PediaCare Triaminic Benadryl Diphenhydramine Pediatan Trinalin Bentyl Dramamine Periactin Tylenol (any but plain) Benztropin Drixoral Polyhistine Unisom Biohist Durahist Pyribenzamine Vicks Bonine Duratan Rescon Vistacot Bromfed Duravent Restall Vistaril IM Brompheniramine Dytan Robitussin Xyzal Carbinoxamine Excedrin PM Rondec Zyrtec ANTI-INFLAMMATORY Anti-inflammatory medications must be stopped 7 days prior to allergy testing Aspirin Celebrex Motrin Aleve Naprosyn (Naproxen) Flexeril Advil Excedrin (PM & Cold/Sinus) Ibuprofen Alka-Seltzer Prednisone Vioxx Meloxicam cold medications BETA BLOCKERS Beta blocker drugs need to be stopped 7 days prior to allergy testing. DO NOT STOP WITHOUT PRESCRIBING PROVIDERS AUTHORIZATION! ORAL COMBINATION PRODUCTS Brand Name Generic Name Brand Name Generic Name Apo-Metoprolol metoprolol Cobetaloc metroprolol/HCTZ Apo-Propranolol propranolol Corzide Nadolol Betaloc metoprolol Inderide propranolol/HCTZ Betapace/AF sotalol -

Prior Authorization PDL Implementation Schedule
Prior Authorization PDL Implementation Schedule Effective Date: Item January 1, 2017 – Descriptive Therapeutic Class Drugs on PDL Drugs which Require PA Nbr Updated: October 1, 2017 1 ADD/ADHD Amphetamine Salt Combo Tablet ( Generic Adderall®) Amphetamine ODT (Adzenys® XR ODT) Stimulants and Related Agents Amphetamine Salt Combo ER (Adderall XR®) Amphetamine Suspension (Dyanavel XR®) Atomoxetine Capsule (Strattera®) Amphetamine Salt Combo ER (Generic; Authorized Generic for Adderall XR) Dexmethylphenidate (Generic; Authorized Generic of Focalin®) Amphetamine Sulfate Tablet (Evekeo®) Dexmethylphenidate ER (Focalin XR®) Armodafinil Tablet (Generic; Authorized Generic; Nuvigil®) Dextroamphetamine Solution (Procentra®) Clonidine ER Tablet (Generic; Kapvay®) Dextroamphetamine Sulfate Tablet (Generic) Dexmethylphenidate (Focalin®) Guanfacine ER Tablet (Generic) Dexmethylphenidate XR (Generic; Authorized Generic for Focalin XR) Lisdexamfetamine Capsule (Vyvanse®) Dextroamphetamine Sulfate Capsule ER (Generic; Dexedrine®Spansule) Methylphenidate IR (Generic) Dextroamphetamine IR Tablet (Zenzedi®) Methylphenidate ER Chew (Quillichew ER®) Dextroamphetamine Solution (Generic for Procentra®) Methylphenidate ER Capsule (Metadate CD®) Guanfacine ER Tablet (Intuniv®) Methylphenidate ER Tablet (Generic; Generic Concerta®; Methamphetamine (Generic; Desoxyn®) Authorized Generic Concerta®) Methylphenidate ER Susp (Quillivant XR®) Methylphenidate IR (Ritalin®) Modafinil Tablet (Generic) Methylphenidate Solution (Generic; Authorized Generic; Methylin®) Methylphenidate -

Instructions for Skin Testing Adult
Patient Instructions for Allergy Testing Yoon Nofsinger, M.D. 3450 East Fletcher Avenue, Tampa, FL 33613, (813) 972-3353 1. If you are scheduled for skin testing, please wear a sleeveless shirt since testing is performed on the arms and sometimes on the back. If you are having an oral challenge test, please do not eat anything at least 1 hour prior to testing. 2. Do not take antihistamines for 7 days and antidepressants for 14 days prior to testing date. See attached list of medications. Please be careful with over the counter sinus and allergy medications. Contact your prescribing physician first before discontinuing any antidepressants. 3. Notify allergy assistant if you have taken oral steroids within the last four weeks. You should not use any steroid cream on the arms or back prior to testing. 5. You may not undergo allergy testing if you are pregnant. 7. You MAY undergo allergy testing if you currently take a Beta Blocker. Please be aware that you will not be able to receive allergy shots if you are on a Beta Blocker, but you may receive allergy drop therapy. Please contact your prescribing physician to see if it is possible to change your medication. 8. You may not undergo allergy testing or treatment if you are taking a MAO Inhibitor, which are typically prescribed for Parkinson’s and depression (Marplan, Nardil, Parnate and Eldepryl). 8. Please note that you must call us at least 4 days prior to testing date to reschedule your appointment or you will be charged a $50 no show fee.