Value-Priced Medication List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Allergy Testing and History Form
Skin Testing Information and Consent 1. Skin Testing An allergy skin test is used to identify the substances that are causing your allergy symptoms. We will apply several extracts of common allergens to the skin and observe for a reaction. The reactions are then graded and confirmatory intradermal testing may be performed. This involves placing a small amount of extract under the skin of the upper arm. We then observe the reaction and record the results. On the day of testing please wear a short-sleeved shirt that can be pushed up comfortably to your shoulder. Allow 1-2 hours for your test session. You will need to stay on the premises during this time. Please do not bring children to your appointment. 2. Risks of Skin Testing Bleeding and infection may occur due to abrading of the skin. Any time the skin integrity is broken it puts on at risk for infection. However, this is a rare occurrence. The antigens used for testing are sterile and approved by the FDA. Occasionally, skin testing can trigger a severe allergic reaction requiring treatment with medications and/or treatment in the ER. Patients with asthma are at increased risk for triggering an asthma attack during testing. You should not undergo testing if you feel that you have allergy or asthma symptoms are currently under poor control. 3. Contraindications to Skin Testing Women who are pregnant or anyone who is currently taking Beta-Blockers, Tricyclic Anti-depressants or MAOI’s medications will NOT be skin tested. Please be sure to inform us of ALL your medications before the skin test is applied. -

Attachment: Extract from Clinical Evaluation Bosentan
AusPAR Attachment 1 Extract from the Clinical Evaluation Report for Bosentan Proprietary Product Name: Tracleer Sponsor: Actelion Pharmaceuticals Australia Pty Ltd First round report: 25 October 2016 Second round report: 3 February 2017 Therapeutic Goods Administration About the Therapeutic Goods Administration (TGA) · The Therapeutic Goods Administration (TGA) is part of the Australian Government Department of Health, and is responsible for regulating medicines and medical devices. · The TGA administers the Therapeutic Goods Act 1989 (the Act), applying a risk management approach designed to ensure therapeutic goods supplied in Australia meet acceptable standards of quality, safety and efficacy (performance), when necessary. · The work of the TGA is based on applying scientific and clinical expertise to decision- making, to ensure that the benefits to consumers outweigh any risks associated with the use of medicines and medical devices. · The TGA relies on the public, healthcare professionals and industry to report problems with medicines or medical devices. TGA investigates reports received by it to determine any necessary regulatory action. · To report a problem with a medicine or medical device, please see the information on the TGA website <https://www.tga.gov.au>. About the Extract from the Clinical Evaluation Report · This document provides a more detailed evaluation of the clinical findings, extracted from the Clinical Evaluation Report (CER) prepared by the TGA. This extract does not include sections from the CER regarding product documentation or post market activities. · The words [Information redacted], where they appear in this document, indicate that confidential information has been deleted. · For the most recent Product Information (PI), please refer to the TGA website <https://www.tga.gov.au/product-information-pi>. -

LOKELMA Is Sodium Zirconium Cyclosilicate, a Potassium Binder
HIGHLIGHTS OF PRESCRIBING INFORMATION • For oral suspension: 10 g per packet (3) These highlights do not include all the information needed to use LOKELMA™ safely and effectively. See full prescribing information for ------------------------------ CONTRAINDICATIONS ---------------------------- LOKELMA™. None. (4) ----------------------- WARNINGS AND PRECAUTIONS --------------------- LOKELMA™ (sodium zirconium cyclosilicate) for oral suspension • Gastrointestinal Adverse Events in Patients with Motility Disorders. Initial U.S. Approval: [2018] (5.1) --------------------------- INDICATIONS AND USAGE ------------------------- • Edema. (5.2) LOKELMA is a potassium binder indicated for the treatment of hyperkalemia in adults. (1) ------------------------------ ADVERSE REACTIONS ---------------------------- Most common adverse reactions with LOKELMA: mild to moderate edema. Limitation of Use (6.1) LOKELMA should not be used as an emergency treatment for life-threatening hyperkalemia because of its delayed onset of action. (1) To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. ---------------------- DOSAGE AND ADMINISTRATION --------------------- • Recommended starting dose is 10 g administered three times a day for ------------------------------ DRUG INTERACTIONS ---------------------------- up to 48 hours. (2.1) In general, other oral medications should be administered at least 2 hours • For maintenance treatment, recommended dose is 10 g once daily. (2.1) before -

Supporting Information a Analysed Substances
Electronic Supplementary Material (ESI) for Analyst. This journal is © The Royal Society of Chemistry 2020 List of contents: Tab. A1 Detailed list and classification of analysed substances. Tab. A2 List of selected MS/MS parameters for the analytes. Tab. A1 Detailed list and classification of analysed substances. drug of therapeutic doping agent analytical standard substance abuse drug (WADA class)* supplier (+\-)-amphetamine ✓ ✓ S6 stimulants LGC (+\-)-methamphetamine ✓ S6 stimulants LGC (+\-)-3,4-methylenedioxymethamphetamine (MDMA) ✓ S6 stimulants LGC methylhexanamine (4-methylhexan-2-amine, DMAA) S6 stimulants Sigma cocaine ✓ ✓ S6 stimulants LGC methylphenidate ✓ ✓ S6 stimulants LGC nikethamide (N,N-diethylnicotinamide) ✓ S6 stimulants Aldrich strychnine S6 stimulants Sigma (-)-Δ9-tetrahydrocannabinol (THC) ✓ ✓ S8 cannabinoids LGC (-)-11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THC-COOH) S8 cannabinoids LGC morphine ✓ ✓ S7 narcotics LGC heroin (diacetylmorphine) ✓ ✓ S7 narcotics LGC hydrocodone ✓ ✓ Cerillant® oxycodone ✓ ✓ S7 narcotics LGC (+\-)-methadone ✓ ✓ S7 narcotics Cerillant® buprenorphine ✓ ✓ S7 narcotics Cerillant® fentanyl ✓ ✓ S7 narcotics LGC ketamine ✓ ✓ LGC phencyclidine (PCP) ✓ S0 non-approved substances LGC lysergic acid diethylamide (LSD) ✓ S0 non-approved substances LGC psilocybin ✓ S0 non-approved substances Cerillant® alprazolam ✓ ✓ LGC clonazepam ✓ ✓ Cerillant® flunitrazepam ✓ ✓ LGC zolpidem ✓ ✓ LGC VETRANAL™ boldenone (Δ1-testosterone / 1-dehydrotestosterone) ✓ S1 anabolic agents (Sigma-Aldrich) -

Hormones in Pregnancy
SYMPOSIUM Hormones in pregnancy Pratap Kumar, Navneet Magon1 Department of Obstetrics and Gynecology, Kasturba Medical College, Manipal University, Manipal, Karnataka, 1Air Force Hospital, Nathu Singh Road, Kanpur Cantt, Uttar Pradesh, India ABSTRACT The endocrinology of human pregnancy involves endocrine and metabolic changes that result from physiological alterations at the boundary between mother and fetus. Progesterone and oestrogen have a great role along with other hormones. The controversies of use of progestogen and others are discussed in this chapter. Progesterone has been shown to stimulate the secretion of Th2 and reduces the secretion of Th1 cytokines which maintains pregnancy. Supportive care in early pregnancy is associated with a significant beneficial effect on pregnancy outcome. Address for correspondence: Prophylactic hormonal supplementation can be recommended for all assisted reproduction Dr. Navneet Magon, techniques cycles. Preterm labor can be prevented by the use of progestogen. The route of Head, Department of Obstetrics administration plays an important role in the drug’s safety and efficacy profile in different and Gynecology, Air Force trimesters of pregnancy. Thyroid disorders have a great impact on pregnancy outcome and Hospital, Nathu Singh Road, needs to be monitored and treated accordingly. Method of locating review: Pubmed, scopus Kanpur Cantt, U.P. India. E‑ mail: [email protected] Key words: Oestrogen, hormones, progesterone, thyroid INTRODUCTION about 250 mg/day. Almost all of the progesterone produced by the placenta enters the placenta, contrast to oestrogen. Steroid hormones like progesterone have been extensively Progesterone production is independent of he precursor studied in the literature with controversies in early available, fetal status including the wellbeing. -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented. -
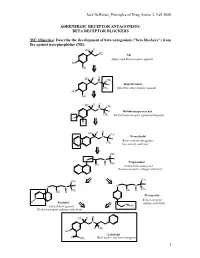
BETA RECEPTOR BLOCKERS MC Objective
Jack DeRuiter, Principles of Drug Action 2, Fall 2000 ADRENERGIC RECEPTOR ANTAGONISTS: BETA RECEPTOR BLOCKERS MC Objective: Describe the development of beta antagonists ("beta blockers") from the agonist norepinephrine (NE): HO H NH2 NE Alpha- and Beta-receptor agonist HO OH H H HO CH N 3 H Isoproterenol CH3 Selective beta-receptor agonist HO OH H H HO CH N 3 H Dichloroisoproterenol CH3 Partial beta-receptor agonist/antagonist Cl Cl H H HO CH N 3 Pronethalol H Beta-receptor antagonist, CH 3 low activity and toxic CH3 O N H Propranolol CH3 OH H Potent beta-antagonist No beta-receptor subtype selectivity CH3 CH3 O N H O N H CH CH OH H 3 OH H 3 Metoprolol N Beta-1-receptor H Pindolol O subtype selectivity Partial beta-agonist CH3 No beta-receptor subtype selectivity HO H H N H CH3 HO Labetolol O NH2 Dual alpha- and beta-antagonst 1 Jack DeRuiter, Principles of Drug Action 2, Fall 2000 MC Objective: Based on their structures, would the beta-blockers be expected to be relatively receptor selective? YES. They do not produce significant blockade of alpha- adrenergic receptors (alpha-1 or alpha-2), histamine receptors, muscarinic receptors or dopamine receptors. MC/PC Objective: Identify which beta blockers are classified as "non-selective": · The “non-selective" classification refers to those beta-blockers capable of blocking BOTH beta-1 and beta-2 receptors with equivalent efficacy. These drugs DO NOT have clinically significant affinity for other neurotransmitter receptors (alpha, dopamine, histamine, acetylcholine, etc.). · ALL of these beta-blockers (except satolol) consist of an aryloxypropanolamine side chain linked to an aromatic or “heteroaromatic” ring which is “ortho” substituted. -

Pharmacology/Therapeutics II Block III Lectures 2013-14
Pharmacology/Therapeutics II Block III Lectures 2013‐14 66. Hypothalamic/pituitary Hormones ‐ Rana 67. Estrogens and Progesterone I ‐ Rana 68. Estrogens and Progesterone II ‐ Rana 69. Androgens ‐ Rana 70. Thyroid/Anti‐Thyroid Drugs – Patel 71. Calcium Metabolism – Patel 72. Adrenocorticosterioids and Antagonists – Clipstone 73. Diabetes Drugs I – Clipstone 74. Diabetes Drugs II ‐ Clipstone Pharmacology & Therapeutics Neuroendocrine Pharmacology: Hypothalamic and Pituitary Hormones, March 20, 2014 Lecture Ajay Rana, Ph.D. Neuroendocrine Pharmacology: Hypothalamic and Pituitary Hormones Date: Thursday, March 20, 2014-8:30 AM Reading Assignment: Katzung, Chapter 37 Key Concepts and Learning Objectives To review the physiology of neuroendocrine regulation To discuss the use neuroendocrine agents for the treatment of representative neuroendocrine disorders: growth hormone deficiency/excess, infertility, hyperprolactinemia Drugs discussed Growth Hormone Deficiency: . Recombinant hGH . Synthetic GHRH, Recombinant IGF-1 Growth Hormone Excess: . Somatostatin analogue . GH receptor antagonist . Dopamine receptor agonist Infertility and other endocrine related disorders: . Human menopausal and recombinant gonadotropins . GnRH agonists as activators . GnRH agonists as inhibitors . GnRH receptor antagonists Hyperprolactinemia: . Dopamine receptor agonists 1 Pharmacology & Therapeutics Neuroendocrine Pharmacology: Hypothalamic and Pituitary Hormones, March 20, 2014 Lecture Ajay Rana, Ph.D. 1. Overview of Neuroendocrine Systems The neuroendocrine -

CARTEOLOL HYDROCHLORIDE- Carteolol Hydrochloride Solution Sandoz Inc ------Carteolol Hydrochloride Ophthalmic Solution USP, 1% Rx Only Sterile
CARTEOLOL HYDROCHLORIDE- carteolol hydrochloride solution Sandoz Inc ---------- Carteolol Hydrochloride Ophthalmic Solution USP, 1% Rx Only Sterile DESCRIPTION Carteolol Hydrochloride Ophthalmic Solution USP, 1% is a nonselective beta-adrenoceptor blocking agent for ophthalmic use. The chemical name for carteolol hydrochloride is (±)-5-[3-[(1,1-dimethylethyl) amino]-2- hydroxypropoxy]-3,4-dihydro-2(1H)-quinolinone monohydrochloride. The structural formula is as follows: C16H24N2O3•HCI Mol. Wt. 328.84 Each mL of sterile solution contains Active: carteolol hydrochloride 10 mg (1%). Preservative: benzalkonium chloride 0.05 mg (0.005%). Inactives: sodium chloride, monobasic and dibasic sodium phosphate, sodium hydroxide and/or hydrochloric acid (to adjust pH to 6.0 - 8.0) and purified water. CLINICAL PHARMACOLOGY Carteolol is a nonselective beta-adrenergic blocking agent with associated intrinsic sympathomimetic activity and without significant membrane-stabilizing activity. Carteolol Hydrochloride reduces normal and elevated intraocular pressure (IOP) whether or not accompanied by glaucoma. The exact mechanism of the ocular hypotensive effect of beta-blockers has not been definitely demonstrated. In general, beta-adrenergic blockers reduce cardiac output in patients in good and poor cardiovascular health. In patients with severe impairment of myocardial function, beta-blockers may inhibit the sympathetic stimulation necessary to maintain adequate cardiac function. Beta-adrenergic blockers may also increase airway resistance in the bronchi and bronchioles due to unopposed parasympathetic activity. Given topically twice daily in controlled domestic clinical trials ranging from 1.5 to 3 months, Carteolol Hydrochloride produced a median percent reduction of IOP 22% to 25%. No significant effects were noted on corneal sensitivity, tear secretion, or pupil size. -

Predictive Model of Bosentan-Induced Liver Toxicity in Japanese Patients with Pulmonary Arterial Hypertension
Canadian Journal of Physiology and Pharmacology Predictive model of bosentan-induced liver toxicity in Japanese patients with pulmonary arterial hypertension Journal: Canadian Journal of Physiology and Pharmacology Manuscript ID cjpp-2019-0656.R1 Manuscript Type: Article Date Submitted by the 24-Mar-2020 Author: Complete List of Authors: Yorifuji, Kennosuke; Shinko Hospital Uemura, Yuko; Shinko Hospital Horibata, Shinji; Shinko Hospital Tsuji, Goh; Shinko Hospital Suzuki, Yoko;Draft Kobe Pharmaceutical University, Clinical Pharmaceutical Science Nakayama, Kazuhiko; Shinko Hospital Hatae, Takashi; Kobe Pharmaceutical University Kumagai, Shunichi; Shinko Hospital EMOTO, Noriaki; Kobe Pharmaceutical University, Clinical Pharmaceutical Science; Kobe University Graduate School of Medicine School of Medicine, Division of Cardiovascular Medicine Is the invited manuscript for consideration in a Special ET-16 Kobe 2019 Issue: bosentan, pulmonary arterial hypertension, pharmacogenetics, CHST3, Keyword: CHST13 https://mc06.manuscriptcentral.com/cjpp-pubs Page 1 of 13 Canadian Journal of Physiology and Pharmacology Predictive model of bosentan-induced liver toxicity in Japanese patients with pulmonary arterial hypertension Kennosuke Yorifuji, M.Pharm.1, 2, 3; Yuko Uemura2; Shinji Horibata, M.Pharm.2, 3; Goh Tsuji, M.D., PhD.2, 4; Yoko Suzuki, M.Pharm.1, 5; Kazuhiko Nakayama, M.D., PhD.6; Takashi Hatae, PhD.7; Shunichi Kumagai, M.D., PhD.2, 4; Noriaki Emoto, M.D., PhD.1, 5 1Laboratory of Clinical Pharmaceutical Science, 7 Education and Research Center -

San Diego ENT
San Diego ENT Allergy Skin Testing Instructions Make sure you review all of your medications with your doctor or the medical assistant when you are scheduled for your allergy test. DON’T’S: • Do note take over-the-counter antihistamines, cold medication, or cough syrup for 10 days prior to the test. This includes Benadryl, Claritin, Zyrtec, Allegra, loratadine, cetirizine, and fexofenadine, Tavist, Dramamine,Atarax, and others. • Do not take prescription antihistamines for 10 days prior to the test including Claritin, Zyrtec, Allegra, loratadine, cetirizine, fexofenadine, and Astelin nasal spray. Also stop antihistamine eye drops 10 days prior to testing. • Do not take beta blockers 5 days prior to testing. These include labetalol, metoprolol, carvedilol, and their brand name equivalents. • Do not take anti-acid medication for 48 hours prior to testing including Zantac, Pepcid, and Tagamet. You may continue to take proton pump inhibitors such as Prilosec, Nexium, Prevacid, Protonix, and Aciphex. • Do not take any sleeping medications for 48 hours prior to testing including Tylenol PM and Excedrin PM. DO’S: • Wear something comfortable that will allow access to your back or both upper arms on the day of testing. • You may continue to use nasal steroid sprays such as Flonase, Nasonex, Nasacort, and Rhinocort. Do not use Astelin. • Review the list of medications that need to be avoided below. Antihistamines to stop 10 days prior to testing: Generic Brand Name Acrivastine Semprex Azatadine Optimine, Trinalin Bropheniramine AccuHist, Bromfed, -

Drugs Affectin the Autonomic Nervous System
Fundamentals of Medical Pharmacology Paterson Public Schools Written by Néstor Collazo, Ph.D. Jonathan Hodges, M.D. Tatiana Mikhaelovsky, M.D. for Health and Related Professions (H.A.R.P.) Academy March 2007 Course Description This fourth year course is designed to give students in the Health and Related Professions (H.A.R.P.) Academy a general and coherent explanation of the science of pharmacology in terms of its basic concepts and principles. Students will learn the properties and interactions between chemical agents (drugs) and living organisms for the rational and safe use of drugs in the control, prevention, and therapy of human disease. The emphasis will be on the fundamental concepts as they apply to the actions of most prototype drugs. In order to exemplify important underlying principles, many of the agents in current use will be singled out for fuller discussion. The course will include the following topics: ¾ The History of Pharmacology ¾ Terminology Used in Pharmacology ¾ Drug Action on Living Organisms ¾ Principles of Pharmacokinetics ¾ Dose-Response Relationships ¾ Time-Response Relationships ¾ Human Variability: Factors that will modify effects of drugs on individuals ¾ Effects of Drugs Attributable to Varying Modes of Administration ¾ Drug Toxicity ¾ Pharmacologic Aspects of Drug Abuse and Drug Dependence Pre-requisites Students must have completed successfully the following courses: Biology, Chemistry, Anatomy and Physiology, Algebra I and II Credits: 5 credits Basic Principles of Drug Action Introduction to Pharmacology a. Basic Mechanisms of Drug Actions b. Dose-response relationships c. Drug absorption d. Biotransformation of Drugs e. Pharmacokinetics f. Factors Affecting Drug Distribution g. Drug Allergy and Pharmacogenetics h.