Pulmonary Valve Replacement After Repaired Tetralogy of Fallot
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reoperation for Tricuspid Regurgitation After Total Correction of Tetralogy of Fallot
Original Reoperation for Tricuspid Regurgitation after Total Article Correction of Tetralogy of Fallot Yoshikazu Hachiro, MD, Nobuyuki Takagi, MD, Tetsuya Koyanagi, MD, and Tomio Abe, MD Background: The aim of this study is to review the outcome of reoperation for severe tricus- pid regurgitation after repair of tetralogy of Fallot . Methods: Between 1972 and 2000, 12 patients underwent reoperation on the tricuspid valve after total correction of tetralogy of Fallot. The mean age at the time of reoperation was 17 years (range, 1 to 39 years). The mean interval between the initial correction and the reoperation was 7.8 years (range, 10 days to 19 years). The functional class was New York Heart Association class II in 2 patients and class III or IV in 10. Six patients underwent tricuspid valve repair, and the others underwent tricuspid valve replacement. Results: Hospital mortality was 16.7% (2/12). Three patients (30%, 3/10) required a second reoperation 1.6, 9.2, and 15.6 years after the most recent reoperation with no deaths. The rea- sons for second reoperation were failure of the tricuspid valve repair in two and a thrombosed valve in one. There were two late deaths. Mean overall event-free actuarial survival at 10 years was 46.3%. Conclusion: Reoperation for severe tricuspid regurgitation after total correction of tetralo- gy of Fallot was associated with a high operative mortality and disappointing long-term results. Tricuspid regurgitation after corrective surgery for tetralogy of Fallot must be diag- nosed promptly and cured, as tolerance is poor because of postoperative right ventricular insufficiency. -
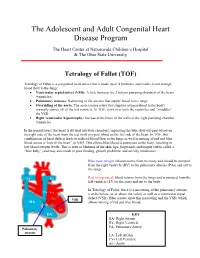
Tetralogy of Fallot (TOF)
The Adolescent and Adult Congenital Heart Disease Program The Heart Center at Nationwide Children’s Hospital & The Ohio State University Tetralogy of Fallot (TOF) Tetralogy of Fallot is a congenital heart defect that is made up of 4 problems and results in not enough blood flow to the lungs: • Ventricular septal defect (VSD): A hole between the 2 bottom pumping chambers of the heart (ventricles) • Pulmonary stenosis: Narrowing of the arteries that supply blood to the lungs • Overriding of the aorta: The aorta (major artery that supplies oxygen blood to the body) normally comes off of the left ventricle. In TOF, it sits over both the ventricles and “straddles” the VSD. • Right ventricular hypertrophy: Increased thickness of the walls of the right pumping chamber (ventricle) In the normal heart, the heart is divided into four chambers, separating the blue (low-oxygen) blood on the right side of the heart from the red (with oxygen) blood on the left side of the heart. In TOF, this combination of heart defects leads to reduced blood flow to the lungs as well as mixing of red and blue blood across a “hole in the heart” or VSD. This allows blue blood to pump out to the body, resulting in low blood oxygen levels. This is seen as blueness of the skin, lips, fingernails and tongue (often called a “blue baby”) and may also result in poor feeding, growth problems, and activity intolerance. Blue (low-oxygen) blood returns from the body and should be pumped from the right ventricle (RV) to the pulmonary arteries (PAs) and out to Aorta the lungs. -
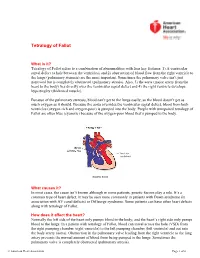
Before Reading the Specific Defect
Tetralogy of Fallot What is it? Tetralogy of Fallot refers to a combination of abnormalities with four key features: 1) A ventricular septal defect (a hole between the ventricles) and 2) obstruction of blood flow from the right ventricle to the lungs (pulmonary stenosis) are the most important. Sometimes the pulmonary valve isn’t just narrowed but is completely obstructed (pulmonary atresia). Also, 3) the aorta (major artery from the heart to the body) lies directly over the ventricular septal defect and 4) the right ventricle develops hypertrophy (thickened muscle). Because of the pulmonary stenosis, blood can’t get to the lungs easily, so the blood doesn’t get as much oxygen as it should. Because the aorta overrides the ventricular septal defect, blood from both ventricles (oxygen-rich and oxygen-poor) is pumped into the body. People with unrepaired tetralogy of Fallot are often blue (cyanotic) because of the oxygen-poor blood that’s pumped to the body. What causes it? In most cases, the cause isn’t known although in some patients, genetic factors play a role. It’s a common type of heart defect. It may be seen more commonly in patients with Down syndrome (in association with AV canal defects) or DiGeorge syndrome. Some patients can have other heart defects along with tetralogy of Fallot. How does it affect the heart? Normally the left side of the heart only pumps blood to the body, and the heart’s right side only pumps blood to the lungs. In a patient with tetralogy of Fallot, blood can travel across the hole (VSD) from the right pumping chamber (right ventricle) to the left pumping chamber (left ventricle) and out into the body artery (aorta). -
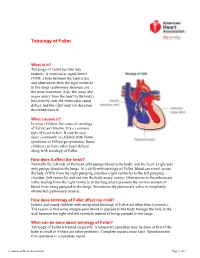
Tetralogy of Fallot
Tetralogy of Fallot What is it? Tetralogy of Fallot has four key features. A ventricular septal defect (VSD; a hole between the ventricles) and obstruction from the right ventricle to the lungs (pulmonary stenosis) are the most important. Also, the aorta (the major artery from the heart to the body) lies directly over the ventricular septal defect, and the right ventricle develops thickened muscle. What causes it? In most children, the cause of tetralogy of Fallot isn’t known. It’s a common type of heart defect. It may be seen more commonly in children with Down syndrome or DiGeorge syndrome. Some children can have other heart defects along with tetralogy of Fallot. How does it affect the heart? Normally the left side of the heart only pumps blood to the body, and the heart’s right side only pumps blood to the lungs. In a child with tetralogy of Fallot, blood can travel across the hole (VSD) from the right pumping chamber (right ventricle) to the left pumping chamber (left ventricle) and out into the body artery (aorta). Obstruction in the pulmonary valve leading from the right ventricle to the lung artery prevents the normal amount of blood from being pumped to the lungs. Sometimes the pulmonary valve is completely obstructed (pulmonary atresia). How does tetralogy of Fallot affect my child? Infants and young children with unrepaired tetralogy of Fallot are often blue (cyanotic). The reason is that some oxygen-poor blood is pumped to the body through the hole in the wall between the right and left ventricle instead of being pumped to the lungs. -
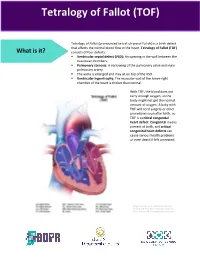
Tetralogy of Fallot (TOF)
Tetralogy of Fallot (TOF) Tetralogy of Fallot (pronounced te-tral-uh-jee of Fal-oh) is a birth defect that affects the normal blood flow in the heart. Tetralogy of Fallot (TOF) What is it? consists of four defects: . Ventricular septal defect (VSD): An opening in the wall between the two lower chambers. Pulmonary stenosis: A narrowing of the pulmonary valve and main pulmonary artery. The aorta is enlarged and may sit on top of the VSD. Ventricular hypertrophy: The muscular wall of the lower-right chamber of the heart is thicker than normal. With TOF, the blood does not carry enough oxygen, so the body might not get the normal amount of oxygen. A baby with TOF will need surgery or other procedures soon after birth, so TOF is a critical congenital heart defect. Congenital means present at birth, and critical congenital heart defects can cause serious health problems or even death if left untreated. Image courtesy of the Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities About 1 in every 2,518 babies is born with TOF. That’s about 1,660 How common is it? babies each year in the United States. The cause of TOF for most babies is unknown. There may be many factors that cause TOF, but more research is needed to understand the What causes it? exact cause. TOF can be diagnosed during pregnancy or after. During pregnancy screenings are done to check for birth defects. After birth a doctor will do a physical examination to see whether the baby has blue-colored How is it diagnosed? skin and lips, called cyanosis. -

Tetralogy of Fallot.” These Podcasts Are Designed to Give Medical Students an Overview of Key Topics in Pediatrics
PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on “Tetralogy of Fallot.” These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio versions are accessible on iTunes or at www.pedcases.com/podcasts. Tetralogy of Fallot Developed by Katie Girgulis, Dr. Andrew Mackie, and Dr. Karen Forbes for PedsCases.com. April 14, 2017 Introduction Hello, my name is Katie Girgulis and I am a medical student at the University of Alberta. This podcast was developed with the help of Dr. Andrew Mackie and Dr. Karen Forbes. Dr. Mackie is a pediatric cardiologist at the Stollery Children’s Hospital, and Dr. Forbes is a pediatrician and medical educator at the Stollery Children’s Hospital. This podcast is about the cardiac condition Tetralogy of Fallot (ToF). For teaching on the general approach to pediatric heart murmurs, please check out the ‘Evaluation of a Heart Murmur’ podcast on Pedscases.com. Slide 2 Learning Objectives By the end of this podcast, the learner will be able to: 1) Recognize the clinical presentations of ToF 2) Describe the four anatomical characteristics of ToF 3) Describe the pathophysiology of the murmur in ToF 4) Formulate initial steps when ToF is suspected 5) Delineate the treatment of hypercyanotic episodes 6) Summarize the definitive treatment for ToF Slide 3 Case – Baby Josh Let’s start with a clinical case: You are working with Dr. Smith, a family physician, during your family medicine rotation. Josh is a 4-month-old infant who is here for a well-baby check. -

Tetralogy of Fallot, Pulmonary Valve Stenosis, Ventricular Septa1 Defect, and Hypertrophic Cardiomyopathy in WKY/Ncrj Rats
003 1-3998/90/2705-0483$02.00/0 PEDIATRIC RESEARCH Vol. 27, No. 5, 1990 Copyright 0 1990 International Pediatric Research Foundation, Inc Prinled in U.S. A Tetralogy of Fallot, Pulmonary Valve Stenosis, Ventricular Septa1 Defect, and Hypertrophic Cardiomyopathy in WKY/NCrj Rats TOSI-IIRO KURIBAYASHI, KAZUTOSHI SHIMOO, TAKASHI NAKAMURA, HIROFUMI TANIWAKI, KENJI HAMAOKA, MASAO NAKAGAWA, YASUHIKO IBATA. TOMOHIKO KOMEDA, AND AKINOBU NAGAOKA Second Department of Anatomy [T.Ku., Y.I.], Second Department of Medicine(K.S., T.N., M.N.], Third Department of Medicine (H. T.], and Children's Research Hospital [K.H.], Kyoto Prefectural University of Medicine, Kyoto, and Central Research Division [TKO.,A.N.] Takeda Chemical Industries Ltd., Osaka, Japan ABSTRACT. We examined anatomically the hearts of 198 (1 1); they have been considered animal models for the human WKY/NCrj rats of 20 litters. There were 51 rats with congenital heart diseases. moderate to severe thickening of the pulmonary valve and Our report describes these cardiac anomalies that occur spon- 19 rats with a ventricular septal defect; the two lesions taneously in rats of an inbred strain. We have already reported occurred together in 16 rats, in 15 of which there were that various myocardial abnormalities similar to human HCM overriding of the aorta, stenosis of the pulmonary outflow occur in the majority of these animals, e.g. cardiac hypertrophy, tract, and hypertrophy of the right ventricle, fulfilling the DST, muscle fiber disarrangement, myocardial fibrosis, coronary criteria for tetralogy of Fallot in man. The papillary muscle arterial wall thickening, and/or narrow left ventricular cavity of the conus was absent in 65 rats. -

CYANOTIC CONGENITAL HEART DISEASE by JAMES W
.-51.I Postgrad Med J: first published as 10.1136/pgmj.25.289.511 on 1 November 1949. Downloaded from CYANOTIC CONGENITAL HEART DISEASE By JAMES W. BROWN, M.D., F.R.C.P. Physician, General Hospital, Grimsby, and Grimsby and Lindsey Rheumatism and Heart Clinics The last 15 years have witnessed a very re- it was possible to build up a clinical picture and so markable change in the attitude of the clinician arrive at a reasonably correct anatomical diagnosis. towards cyanotic congenital heart disease. It is At the same time knowledge of the natural history within the memory of many that it was the custom of congenital heart disease has also increased, and to make a simple diagnosis of congenital heart some idea of its prognosis has been ascertained. abnormality with cyanosis, and express the opinion Lastly, the conception of Taussig that an in- that little or nothing could be done about it. Then adequate pulmonary blood supply was the critical there appeared the pioneer work of Abbott and abnormality in many cyanotic cases, and the de- others which produced not only a classification of velopment of an anastomotic operation between the the various abnormalities, but furnished a mass of systemic and pulmonary circulations so as to clinical and postmortem observations from which furnish an artificial ductus arteriosus, by Blalock Protected by copyright. http://pmj.bmj.com/ on September 23, 2021 by guest. FIG. i.-Tetralogy of Fallot. Female aged 3 years. Right ventricle opened. An arrow marks the subvalvular stenosis. of the pulmonary artery. Ventricular septal defect with probe passing into it behind the crista supraventricularis. -

Dyspnea in Adult Patient with Corrected Tetralogy of Fallot
Dyspnea in adult patient with corrected Tetralogy of Fallot F. Mut, M. Beretta Nuclear Medicine Service, Asociacion Española Montevideo, Uruguay Clinical history • Male 51 y.o. • Tetralogy of Fallot (TF) - acyanotic form. • Operated 16 years before • Dyspnea. • EKG: LV, RV hypertrophy, repolarization changes. • Echo: LVH, RVH with preserved systolic function of both ventricles, mild pulmonary valve stenosis. • MPS was indicated to rule out associated CAD. Clinical history • Exercise/rest MPS with 99mTc-MIBI was performed. • 90% of maximum predicted heart rate was achieved. • No ECG changes, no chest pain. • Dyspnea. Myocardial perfusion study STRESS REST STRESS REST STRESS REST Myocardial perfusion (enlarged stress images) The MPI results indicate: a) Normal study. b) LV & RV hypertrophy, anterior ischemia. c) LV & RV hypertrophy, transient dilation. d) LV & RV hypertrophy, inferior ischemia. The MPI results indicate: a) Normal study. b) LV & RV hypertrophy, anterior ischemia. c) LV & RV hypertrophy, transient dilation. d) LV & RV hypertrophy, inferior ischemia. • There is very marked LV & RV hypertrophy. • No perfusion defects are observed. • The LV cavity seems to be larger in the post-stress study as compared to rest, indicating transient ischemic dilation (TID). The 4 features typical of Tetralogy of Fallot include: a) Right ventricular outflow tract obstruction, ventricular septal defect, dextroposition of the aorta, and right ventricular hypertrophy. b) Right ventricular outflow tract obstruction, atrial septal defect, dextrocardia, and left ventricular hypertrophy. c) Mitral valve prolapse, ventricular septal defect, dextroposition of the aorta, and left ventricular hypertrophy. d) Right ventricular outflow tract obstruction, atrial septal defect, dextrocardia, and right hypertrophy. The 4 features typical of Tetralogy of Fallot include: a) Right ventricular outflow tract obstruction, ventricular septal defect, dextroposition of the aorta, and right ventricular hypertrophy. -

Tetralogy of Fallot: Basic Imaging Findings and Management
Journal of Radiology Nursing 38 (2019) 164e167 Contents lists available at ScienceDirect Journal of Radiology Nursing journal homepage: www.sciencedirect.com/journal/ journal-of-radiology-nursing Tetralogy of Fallot: Basic Imaging Findings and Management * Ramya Vajapey, MD, David Majdalany, MD Division of Clinical Cardiology, Department of Heart and Vascular Institute, Cleveland Clinic Foundation, Cleveland, Ohio abstract Keywords: Tetralogy of Fallot (TOF) is one of the most common cyanotic, congenital heart disease with complex Tetralogy of Fallot anatomic malformations and unknown etiology. With medical advancements, most patients in this Congenital cohort require early total correction and survive into adulthood. This review addresses various imaging Malformation modalities that help diagnose TOF, methods to correct and repair TOF-associated cardiac malformations, Right ventricular hypertrophy and the sequelae and long-term complications of this disease. © 2019 Association for Radiologic & Imaging Nursing. Published by Elsevier Inc. All rights reserved. Background Imaging findings Tetralogy of Fallot (TOF) is one of the most common causes of Electrocardiogram/Chest X-ray cyanotic cardiac disease and occurs in 3 of every 10,000 births, ac- counting for about 10% of all congenital heart diseases. TOF is a Initial evaluation of TOF includes obtaining an electrocardio- cardiac malformation that has a ventricular septal defect, obstruc- gram, which will typically demonstrate right axis deviation with tion of right ventricular outflow tract, aortic root overriding the right bundle branch block and right ventricular hypertrophy with ventricular septum, and right ventricular hypertrophy (see Figure 1) large R waves in anterior precordial leads and large S wave in the (Bailliard & Anderson, 2009). It was first described by Danish lateral precordial leads. -
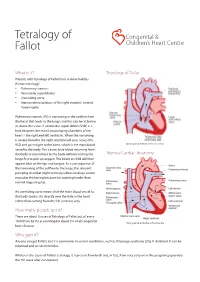
Tetralogy of Fallot
Tetralogy of Congenital & Fallot Children’s Heart Centre What is it? Tetralogy of Fallot Patients with Tetralogy of Fallot have 4 abnormalities (hence tetralogy): • Pulmonary stenosis • Ventricular septal defect • Overriding aorta • Increased musculature of the right ventricle termed hypertrophy. Pulmonary stenosis (PS) is narrowing in the outflow from the heart that leads to the lungs, and this can be at, below or above the valve. A ventricular septal defect (VSD) is a hole between the main two pumping chambers of the heart – the right and left ventricles. When the narrowing is severe, blood in the right ventricle will pass across this VSD and go straight to the aorta, which is the main blood © Congenital & Children’s Heart Centre vessel to the body. This causes blue blood returning from the body to return back to the body without visiting the Normal Cardiac Anatomy lungs first to pick up oxygen. The infant or child will then appear blue on the lips and tongue. As a consequence of the narrowing of the outflow to the lungs, the relevant pumping chamber (right ventricle) often develops severe muscular thickening because it is working harder than normal (hypertrophy). An overriding aorta means that the main blood vessel to the body (aorta) sits directly over the hole in the heart rather than coming from the left ventricle only. How many people get it? There are about 5 cases of Tetralogy of Fallot out of every 10,000 live births accounting for about 5% of all congenital © Congenital & Children’s Heart Centre heart disease. Who gets it? Anyone can get Fallot’s but it is commoner in certain conditions, such as DiGeorge syndrome (22q11 deletion). -

MRI and Computed Tomography of Cardiac and Pulmonary Complications of Tetralogy of Fallot in Adults
PICTORIAL ESSAY MRI and Computed Tomography of Cardiac and Pulmonary Complications of Tetralogy of Fallot in Adults Robyn D. Gartner, MD,* Nicole J. Sutton, MD,w Samuel Weinstein, MD,z Hugo Spindola-Franco, MD,* and Linda B. Haramati, MD, MS* Since the introduction of surgical palliation in the Abstract: Tetralogy of Fallot (TOF) represents the most common 1940s and repair in the 1970s, there has developed an incre- form of cyanotic congenital heart disease, accounting for 6.8% of asingly large population of adults living with TOF. The all congenital heart disease. As surgical techniques and medical growing population of adults with TOF merits increased management of patients with TOF have improved, most affected attention to their unique problems. The imaging features patients are reaching adulthood. Though surgical outcomes are mirror the evolution in surgical techniques. Radiologists favorable (<2% early mortality rate), adults with TOF may should be familiar with the defects associated with TOF, experience complications from the long-term sequelae of congenital the types of surgeries performed, and the potential compli- heart disease and complications related to treatment. We describe cations. This paper will focus on the value of computed common and uncommon findings in adults with TOF, including tomography (CT) and magnetic resonance imaging (MRI) pulmonary insufficiency, central and peripheral pulmonary artery in contributing to the care of adults with TOF. stenosis and aneurysms, in addition to graft and shunt-related complications. Pulmonary function abnormalities and lung paren- chymal imaging findings will be detailed. The diagnostic value of computed tomography and magnetic resonance imaging in adults IMAGING MODALITIES with complications of TOF will be illustrated.