Dyspnea in Adult Patient with Corrected Tetralogy of Fallot
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reoperation for Tricuspid Regurgitation After Total Correction of Tetralogy of Fallot
Original Reoperation for Tricuspid Regurgitation after Total Article Correction of Tetralogy of Fallot Yoshikazu Hachiro, MD, Nobuyuki Takagi, MD, Tetsuya Koyanagi, MD, and Tomio Abe, MD Background: The aim of this study is to review the outcome of reoperation for severe tricus- pid regurgitation after repair of tetralogy of Fallot . Methods: Between 1972 and 2000, 12 patients underwent reoperation on the tricuspid valve after total correction of tetralogy of Fallot. The mean age at the time of reoperation was 17 years (range, 1 to 39 years). The mean interval between the initial correction and the reoperation was 7.8 years (range, 10 days to 19 years). The functional class was New York Heart Association class II in 2 patients and class III or IV in 10. Six patients underwent tricuspid valve repair, and the others underwent tricuspid valve replacement. Results: Hospital mortality was 16.7% (2/12). Three patients (30%, 3/10) required a second reoperation 1.6, 9.2, and 15.6 years after the most recent reoperation with no deaths. The rea- sons for second reoperation were failure of the tricuspid valve repair in two and a thrombosed valve in one. There were two late deaths. Mean overall event-free actuarial survival at 10 years was 46.3%. Conclusion: Reoperation for severe tricuspid regurgitation after total correction of tetralo- gy of Fallot was associated with a high operative mortality and disappointing long-term results. Tricuspid regurgitation after corrective surgery for tetralogy of Fallot must be diag- nosed promptly and cured, as tolerance is poor because of postoperative right ventricular insufficiency. -
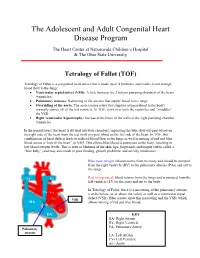
Tetralogy of Fallot (TOF)
The Adolescent and Adult Congenital Heart Disease Program The Heart Center at Nationwide Children’s Hospital & The Ohio State University Tetralogy of Fallot (TOF) Tetralogy of Fallot is a congenital heart defect that is made up of 4 problems and results in not enough blood flow to the lungs: • Ventricular septal defect (VSD): A hole between the 2 bottom pumping chambers of the heart (ventricles) • Pulmonary stenosis: Narrowing of the arteries that supply blood to the lungs • Overriding of the aorta: The aorta (major artery that supplies oxygen blood to the body) normally comes off of the left ventricle. In TOF, it sits over both the ventricles and “straddles” the VSD. • Right ventricular hypertrophy: Increased thickness of the walls of the right pumping chamber (ventricle) In the normal heart, the heart is divided into four chambers, separating the blue (low-oxygen) blood on the right side of the heart from the red (with oxygen) blood on the left side of the heart. In TOF, this combination of heart defects leads to reduced blood flow to the lungs as well as mixing of red and blue blood across a “hole in the heart” or VSD. This allows blue blood to pump out to the body, resulting in low blood oxygen levels. This is seen as blueness of the skin, lips, fingernails and tongue (often called a “blue baby”) and may also result in poor feeding, growth problems, and activity intolerance. Blue (low-oxygen) blood returns from the body and should be pumped from the right ventricle (RV) to the pulmonary arteries (PAs) and out to Aorta the lungs. -

Usefulness Ofcontinuous Positive Airway Pressure in Differential
Arch Dis Child: first published as 10.1136/adc.53.6.456 on 1 June 1978. Downloaded from Archives of Disease in Childhood, 1978, 53, 456-460 Usefulness of continuous positive airway pressure in differential diagnosis of cardiac from pulmonary cyanosis in newborn infants P. SYAMASUNDAR RAO, BRENDA L. MARINO, AND ALEX F. ROBERTSON III From the Department of Pediatrics, Sections of Pediatric Cardiology and Neonatology, Medical College of Georgia, Augusta, Georgia, USA SUMMARY Differential diagnosis of cyanosis in the neonate is difficult and cardiac catheterisa- tion may be required for a correct diagnosis. It has been suggested that the response of Pao2 to continuous positive airway pressure (CPAP) with 100% oxygen may be useful. The purpose of this study was to test further this hypothesis by studying all neonates investigated for cyanosis with a Pao2 -50 torr in 0 8 to 1 .0 F1o2. Arterial blood samples were obtained in an F1o2 of 0 21-0 .4 and 0 8-1 .0, and in an F1O2 of 0 8-1 0 with 8-10 cm CPAP, and were analysed for Pao2, Paco2, and pH, bicarbonate being calculated. The final diagnoses were congenital heart disease (CHD) 21 cases, pulmonary parenchymal disease (PD) 10 cases, and persistent fetal circulation (PFC) 3 cases. No significant difference in pH, bicarbonate, or Paco2 was observed among the three groups or with CPAP. In the CHD and PFC infants CPAP produced no significant change in Pao2. In the PD babies Pao2 increased by an average of 33 torr (P<0 05). Despite thus attaining statistical significance 2 PD infants had no increase in Pao2 with CPAP. -

Pharmacy Policy Statement
PHARMACY POLICY STATEMENT Ohio Medicaid DRUG NAME Synagis (palivizumab) BILLING CODE 90378 (1 unit = 1 vial) BENEFIT TYPE Medical SITE OF SERVICE ALLOWED Office/Outpatient Hospital/Home COVERAGE REQUIREMENTS Prior Authorization Required (Preferred Product) QUANTITY LIMIT— 1 vial per month (max 5 during respiratory syncytial virus season) LIST OF DIAGNOSES CONSIDERED NOT Click Here MEDICALLY NECESSARY Synagis (palivizumab) is a preferred product and will only be considered for coverage under the medical/pharmacy benefit when the following criteria are met: Members must be clinically diagnosed with one of the following disease states and meet their individual criteria as stated. PREVENTION OF RESPIRATORY TRACT DISEASE CAUSED BY RESPIRATORY SYNCYTIAL VIRUS (RSV) For initial authorization: 1. Request must be made during the RSV season (November 1st through March 31st) AND initiation of injections should be timed with the onset of laboratory confirmed cases of RSV activity in the community, no earlier than November 1, 2017; AND 2. Member is < 12 months old at the beginning of the RSV season AND meet one of the following criteria (chart notes must be provided to support evidence): a) Member was born < 29 weeks, 0 days’ gestation; b) Member has Chronic Lung Disease (CLD) of prematurity (defined as gestational age <32 weeks, 0 days and a requirement for >21% oxygen for at least the first 28 days after birth); c) Member has hemodynamically significant Congenital Heart Disease (CHD) with one or more of the following: i) Acyanotic heart disease (e.g. atrial septal defect (ASD), ventricular septal defect (VSD), patent ductus arteriosus (PDA), etc.), AND member is receiving medication to control congestive heart failure (CHF) AND will require cardiac surgical procedures; ii) Moderate to severe pulmonary hypertension; iii) Cyanotic heart defect (e.g. -
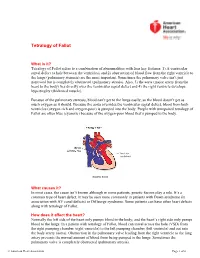
Before Reading the Specific Defect
Tetralogy of Fallot What is it? Tetralogy of Fallot refers to a combination of abnormalities with four key features: 1) A ventricular septal defect (a hole between the ventricles) and 2) obstruction of blood flow from the right ventricle to the lungs (pulmonary stenosis) are the most important. Sometimes the pulmonary valve isn’t just narrowed but is completely obstructed (pulmonary atresia). Also, 3) the aorta (major artery from the heart to the body) lies directly over the ventricular septal defect and 4) the right ventricle develops hypertrophy (thickened muscle). Because of the pulmonary stenosis, blood can’t get to the lungs easily, so the blood doesn’t get as much oxygen as it should. Because the aorta overrides the ventricular septal defect, blood from both ventricles (oxygen-rich and oxygen-poor) is pumped into the body. People with unrepaired tetralogy of Fallot are often blue (cyanotic) because of the oxygen-poor blood that’s pumped to the body. What causes it? In most cases, the cause isn’t known although in some patients, genetic factors play a role. It’s a common type of heart defect. It may be seen more commonly in patients with Down syndrome (in association with AV canal defects) or DiGeorge syndrome. Some patients can have other heart defects along with tetralogy of Fallot. How does it affect the heart? Normally the left side of the heart only pumps blood to the body, and the heart’s right side only pumps blood to the lungs. In a patient with tetralogy of Fallot, blood can travel across the hole (VSD) from the right pumping chamber (right ventricle) to the left pumping chamber (left ventricle) and out into the body artery (aorta). -
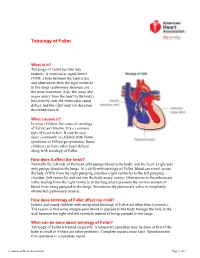
Tetralogy of Fallot
Tetralogy of Fallot What is it? Tetralogy of Fallot has four key features. A ventricular septal defect (VSD; a hole between the ventricles) and obstruction from the right ventricle to the lungs (pulmonary stenosis) are the most important. Also, the aorta (the major artery from the heart to the body) lies directly over the ventricular septal defect, and the right ventricle develops thickened muscle. What causes it? In most children, the cause of tetralogy of Fallot isn’t known. It’s a common type of heart defect. It may be seen more commonly in children with Down syndrome or DiGeorge syndrome. Some children can have other heart defects along with tetralogy of Fallot. How does it affect the heart? Normally the left side of the heart only pumps blood to the body, and the heart’s right side only pumps blood to the lungs. In a child with tetralogy of Fallot, blood can travel across the hole (VSD) from the right pumping chamber (right ventricle) to the left pumping chamber (left ventricle) and out into the body artery (aorta). Obstruction in the pulmonary valve leading from the right ventricle to the lung artery prevents the normal amount of blood from being pumped to the lungs. Sometimes the pulmonary valve is completely obstructed (pulmonary atresia). How does tetralogy of Fallot affect my child? Infants and young children with unrepaired tetralogy of Fallot are often blue (cyanotic). The reason is that some oxygen-poor blood is pumped to the body through the hole in the wall between the right and left ventricle instead of being pumped to the lungs. -

Review Article Congenital Heart Diseases
KYAMC Journal Vol. 9, No.1, April 2018 Review Article Congenital heart diseases: A review of echocardiogram records Md. Saiful Islam1, Md. Moniruzzaman2 Abstract Congenital heart defect (CHD) means an anatomic malformation of the heart or great vessels which occurs during intrauterine development, irrespective of the age at presentation. They can disrupt the normal blood flow through the heart. The blood flow can slow down, go in the wrong direction or to the wrong place, or be blocked completely. Broadly congenital heart defects can be acyanotic and cyanotic. We have reviewed retrospectively from echocardiogram record nearly two years of period & collected total 404 patients with congenital heart defects. Among them 329 (81.43%) was acyanotic and 75 (18.57%) was cyanotic congenital defects with variety of diagnosis. Ventricular septal defect was the most common acyanotic heart defect and Tetralogy of Fallot was the most common cyanotic heart defect. There was no significant gender deference. Keywords: Acyanotic, Congenital heart disease, Cyanotic. Date of received: 11. 11. 2017 Date of acceptance: 05. 01. 2018 Introduction known. The majority of the defects can be explained by Congenital heart defects (CHD) are reported in almost 1% of multifactorial inheritance hypothesis which states that a live births, and about half of these children need medical or predisposed fetus, when exposed to a given environmental surgical management in infancy1. In the first decade, a further trigger, to which the fetus is sensitive during the critical period 25% require surgery to maintain or improve their life1. Only of cardiac morphogenesis may develop the disease5. A variety 10% survive to adolescence without specific treatment. -

Pulmonary-Atresia-Mapcas-Pavsdmapcas.Pdf
Normal Heart © 2012 The Children’s Heart Clinic NOTES: Children’s Heart Clinic, P.A., 2530 Chicago Avenue S, Ste 500, Minneapolis, MN 55404 West Metro: 612-813-8800 * East Metro: 651-220-8800 * Toll Free: 1-800-938-0301 * Fax: 612-813-8825 Children’s Minnesota, 2525 Chicago Avenue S, Minneapolis, MN 55404 West Metro: 612-813-6000 * East Metro: 651-220-6000 © 2012 The Children’s Heart Clinic Reviewed March 2019 Pulmonary Atresia, Ventricular Septal Defect and Major Aortopulmonary Collateral Arteries (PA/VSD/MAPCAs) Pulmonary atresia (PA), ventricular septal defect (VSD) and major aortopulmonary collateral arteries (MAPCAs) is a rare type of congenital heart defect, also referred to as Tetralogy of Fallot with PA/MAPCAs. Tetralogy of Fallot (TOF) is the most common cyanotic heart defect and occurs in 5-10% of all children with congenital heart disease. The classic description of TOF includes four cardiac abnormalities: overriding aorta, right ventricular hypertrophy (RVH), large perimembranous ventricular septal defect (VSD), and right ventricular outflow tract obstruction (RVOTO). About 20% of patients with TOF have PA at the infundibular or valvar level, which results in severe right ventricular outflow tract obstruction. PA means that the pulmonary valve is closed and not developed. When PA occurs, blood can not flow through the pulmonary arteries to the lungs. Instead, the child is dependent on a patent ductus arteriosus (PDA) or multiple systemic collateral vessels (MAPCAs) to deliver blood to the lungs for oxygenation. These MAPCAs usually arise from the de- scending aorta and subclavian arteries. Commonly, the pulmonary arteries are abnormal, with hypoplastic (small and underdeveloped) central and branch pulmonary arteries and/ or non-confluent central pulmonary arteries. -
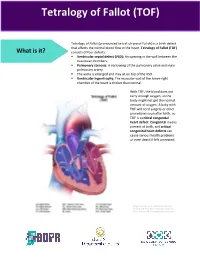
Tetralogy of Fallot (TOF)
Tetralogy of Fallot (TOF) Tetralogy of Fallot (pronounced te-tral-uh-jee of Fal-oh) is a birth defect that affects the normal blood flow in the heart. Tetralogy of Fallot (TOF) What is it? consists of four defects: . Ventricular septal defect (VSD): An opening in the wall between the two lower chambers. Pulmonary stenosis: A narrowing of the pulmonary valve and main pulmonary artery. The aorta is enlarged and may sit on top of the VSD. Ventricular hypertrophy: The muscular wall of the lower-right chamber of the heart is thicker than normal. With TOF, the blood does not carry enough oxygen, so the body might not get the normal amount of oxygen. A baby with TOF will need surgery or other procedures soon after birth, so TOF is a critical congenital heart defect. Congenital means present at birth, and critical congenital heart defects can cause serious health problems or even death if left untreated. Image courtesy of the Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities About 1 in every 2,518 babies is born with TOF. That’s about 1,660 How common is it? babies each year in the United States. The cause of TOF for most babies is unknown. There may be many factors that cause TOF, but more research is needed to understand the What causes it? exact cause. TOF can be diagnosed during pregnancy or after. During pregnancy screenings are done to check for birth defects. After birth a doctor will do a physical examination to see whether the baby has blue-colored How is it diagnosed? skin and lips, called cyanosis. -

Track 5: Cardiology and the Imaging Revolution
TRACK 5: CARDIOLOGY AND THE IMAGING REVOLUTION Volume 10 • Number 1 Abstract no: 1 Summer 2013 Real time 3-D echocardiographic characteristics of left ventricle and left atrium in normal children Bao Phung Tran Cong, Nii Masaki, Miyakoshi Chihiro, Yoshimoto Jun, Kato Atsuko, Ibuki Keichiro, Kim Sunghae, Mitsushita Norie, Tanaka Yasuhiko and Ono Yasuo Cardiac Department, Shizuoka Children’s Hospital, Shizuoka, Japan Background: The accurate assessment of left atrial (LA) and/or left ventricular (LV) volume and contractility is crucial for the management of patients with congenital heart disease. The real time 3-dimensional echocardiography (RT3-DE) is reported to show better correlation with magnetic resonance imaging (MRI) in estimating LV and LA volume than conventional 2-dimensional echocardiography (2-DE). On the other hand, the volume measurement in RT3-DE is also reported to be significantly smaller than those in MRI, necessitating the establishment of normal values of RT3-DE itself. Aim: To identify the normal values of LV and LA volume measured by RT3-DE in Japanese children. Methods: Sixty four normal school students (age: median 9.6 years; range (5.5 - 14.5); male 26, female 38) were enrolled in this study. End-diastolic and end- systolic LV and LA volumes were analysed using M-mode in short-axis view, 2-D biplane method, and RT3-DE. We used IE-33 (PHILIPS) with matrix probe X7 and X4. Off-line assessment to calculate LA and LV volume was done using QLAB 8.1 (Philips). Results: Forty nine children (age: median 9.1 years, range (6 - 14); male 21, female 28) had adequate RT3-DE data sets and were analysed. -

Tetralogy of Fallot.” These Podcasts Are Designed to Give Medical Students an Overview of Key Topics in Pediatrics
PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on “Tetralogy of Fallot.” These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio versions are accessible on iTunes or at www.pedcases.com/podcasts. Tetralogy of Fallot Developed by Katie Girgulis, Dr. Andrew Mackie, and Dr. Karen Forbes for PedsCases.com. April 14, 2017 Introduction Hello, my name is Katie Girgulis and I am a medical student at the University of Alberta. This podcast was developed with the help of Dr. Andrew Mackie and Dr. Karen Forbes. Dr. Mackie is a pediatric cardiologist at the Stollery Children’s Hospital, and Dr. Forbes is a pediatrician and medical educator at the Stollery Children’s Hospital. This podcast is about the cardiac condition Tetralogy of Fallot (ToF). For teaching on the general approach to pediatric heart murmurs, please check out the ‘Evaluation of a Heart Murmur’ podcast on Pedscases.com. Slide 2 Learning Objectives By the end of this podcast, the learner will be able to: 1) Recognize the clinical presentations of ToF 2) Describe the four anatomical characteristics of ToF 3) Describe the pathophysiology of the murmur in ToF 4) Formulate initial steps when ToF is suspected 5) Delineate the treatment of hypercyanotic episodes 6) Summarize the definitive treatment for ToF Slide 3 Case – Baby Josh Let’s start with a clinical case: You are working with Dr. Smith, a family physician, during your family medicine rotation. Josh is a 4-month-old infant who is here for a well-baby check. -

Clinical Presentations of Critical Cardiac Defects in the Newborn: Decision Making and Initial Management
Review article DOI: 10.3345/kjp.2010.53.6.669 Korean J Pediatr 2010;53(6):669-679 Clinical presentations of critical cardiac defects in the newborn: Decision making and initial management Jae Young Lee, M.D. The risk of mortality and morbidity of patients with congenital heart defects (CHDs) is highest during neonatal period and increases Department of Pediatrics, College of Medicine, the when diagnosis and proper management are delayed. Neonates Catholic University of Korea, Seoul, Korea with critical CHDs may present with severe cyanosis, respiratory distress, shock, or collapse, all of which are also frequent clinical presentations of various respiratory problems or sepsis in the newborn. Early diagnosis and stabilization and timely referral to a tertiary cardiac center are crucial to improve the outcomes in Received: 7 May 2010, Accepted: 17 May 2010 neonates with CHDs. In this review, the clinical presentation of Corresponding author: Jae Young Lee, M.D. critical and potentially life-threatening CHDs is discussed along with Department of Pediatrics, College of Medicine, the Catholic University of Korea, 505, Banpo-dong, Seocho-gu, Seoul 137- brief case reviews to help understand the hemodynamics of these 701, Korea defects and ensure proper decision-making in critically ill patients. Tel: +82-2258-6189, Fax: +82-2-537-4544 E-mail: [email protected] Key Words: Congenital heart defect, Ductal-dependent lesions, Copyright © 2010 by The Korean Pediatric Society Cyanosis, Shock Introduction in these critically ill patients. In this review, clinical presentations of potentially life-threatening CHDs in neonates were discussed Congenital heart defects (CHDs) are the most common birth along with brief case reviews to help pediatricians understand the defects with an incidence of approximately 6-8 in 1,000 live births, hemodynamics of these defects and to facilitate correct decision- accounting for 6-10% of all infant deaths and 20-40% of all infant making in these patients.