Aseexam Review Course
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unusual Right Ventricle Aneurysm and Dysplastic Pulmonary Valve With
CASE REPORT Unusual right ventricle aneurysm and dysplastic pulmonary valve with mitral valve hypoplasia Ozge Pamukcu, Abdullah Ozyurt, Mustafa Argun, Ali Baykan, Nazmi Narın, Kazım Uzum Erciyes University School of Medicine, Division of Pediatric Cardiology, Kayseri, Turkey ABSTRACT We report a newborn with an unusual combination of aneurysmally dilated thin‑walled right ventricle with hypertrophy of the apical muscles of the right ventricle. There was narrow pulmonary annulus, pulmonary regurgitation, and hypoplasia of the mitral valve and left ventricle. We propose that this heart represents a partial form of Uhl`s anomaly. Keywords: Absent pulmonary valve, right ventricle aneurysm, Uhl’s anomaly INTRODUCTION A pansystolic murmur was heard. The chest X‑ray showed marked cardiomegaly and a cardiothoracic ratio of 85%. Aneursymal dilatation of the right ventricle can be seen Echocardiography showed an aneurysmally dilated right in several malformations like congenital aneurysm, Uhl’s ventricle [Figure 1, Videos 1‑3]. The entire right ventricle anomaly, arrhythmogenic right ventricular dysplasia, except the apical portion was dilated. There was apical atrialized portion of the Ebstein’s anomaly, absent muscular hypertrophy of the right ventricle. [Figure 1] right pericardium, or post‑ischemic aneurysms.[1] Such The tricuspid valve was mildly hypoplastic (Z score ‑0.95) aneurysms are being increasingly diagnosed prenatally.[2] and prolapsing, but not displaced. The pulmonary valve was dysplastic and doming [Figure 2], with mild We report a case having an aneurysmatically dilated right pulmonary regurgitation [Figure 3]. Although the ventricle resembling the Uhl’s Anomaly, with an unusual pulmonary annulus was not very small, the anatomy combination of lesions. -
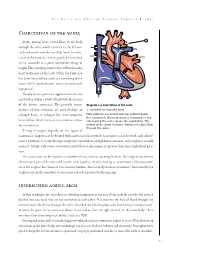
Coarctation of the Aorta Interrupted Aortic Arch
T HE P EDIATRIC C ARDIAC S URGERY I NQUEST R EPORT Coarctation of the aorta In the normal heart, blood flows to the body through the aorta, which connects to the left ven- tricle and arches over the top of the heart.In coarc- tation of the aorta,the aorta is pinched (in medical terms, coarcted) at a point somewhere along its length. This pinching restricts blood flow from the heart to the rest of the body. Often, the baby also has other heart defects, such as a narrowing of the aortic arch (in medical terms,transverse aortic arch hypoplasia). Usually no symptoms are apparent at birth, but can develop within a week of birth with the closure of the ductus arteriosus. The possible conse- Diagram 2.9 Coarctation of the aorta quences of this condition are poor feeding, an 1 – pinched or coarcted aorta enlarged heart, an enlarged liver and congestive Flow patterns are normal but are reduced below the coarctation. Blood pressure is increased in ves- heart failure. Blood pressure also increases above sels leaving the aorta above the coarctation. The the constriction. broken white arrow indicates diminished blood flow through the aorta. Timing of surgery depends on the degree of coarctation. Surgery may be delayed with mild coarctation (which sometimes is not detected until adoles- cence). However, if a baby develops congestive heart failure or high blood pressure, early surgery is usually required. A baby with severe coarctation should have early surgery to prevent long-term high blood pres- sure. The coarctation can be repaired in a number of ways without opening the heart.The surgeon can remove the narrowed part of the aorta and sew the ends together, thereby creating an anastomosis. -

Giant Right Atrium Or Ebstein Anomaly? Hacer Kamalı1, Abdullah Erdem1*, Cengiz Erol2 and Atıf Akçevin3
ISSN: 2378-2951 Kamalı et al. Int J Clin Cardiol 2017, 4:104 DOI: 10.23937/2378-2951/1410104 Volume 4 | Issue 4 International Journal of Open Access Clinical Cardiology CASE REPORT Giant Right Atrium or Ebstein Anomaly? Hacer Kamalı1, Abdullah Erdem1*, Cengiz Erol2 and Atıf Akçevin3 1Department of Pediatric Cardiology, Istanbul Medipol University, Turkey 2Department of Radiology, Istanbul Medipol University, Turkey 3Department of Cardiovascular Surgery, Istanbul Medipol University, Turkey *Corresponding author: Abdullah Erdem, Associate Professor of Pediatric Cardiology, Department of Pediatric Cardiology, Istanbul Medipol University, Istanbul, Turkey, Tel: +905-0577-05805, Fax: +90212467064, E-mail: drabdullaherdem@ hotmail.com partment for exercise intolerance. She had been diag- Abstract nosed and followed up as Ebstein anomaly for ten years. Many acquired and congenital pathologies could cause en- Her heart rate was 75/min with normal jugular venous largement of Right Atrium (RA). In rare pathologies we couldn’t find an obvious reason for enlargement of RA. Sometimes it pressure. Findings of lung examination were normal. leads to misdiagnosis if we try to explain an unknown pa- Auscultation of heart revealed a 2/6 systolic murmur on thology with well known pathology. In this article we de- the lower left edge of the sternum. On abdominal ex- scribe a woman who presented with giant enlargement of amination, there was no organomegaly. Arterial Oxygen the RA misdiagnosed as Ebstein anomaly. Cardiac MRI is highly helpful for differential diagnosis of these kinds of atrial Saturation (SpO2) was 98%. Electrocardiography (ECG) pathologies. Although it is rare, giant RA should be consid- revealed absence of atrial fibrillation, the rhythm was ered in differential diagnosis of huge RA without an obvious sinus but there was right axis deviation and right bundle cause. -

Maternal and Fetal Outcomes of Admission for Delivery in Women with Congenital Heart Disease
Supplementary Online Content Hayward RM, Foster E, Tseng ZH. Maternal and fetal outcomes of admission for delivery in women with congenital heart disease. JAMA Cardiol. Published online April 12, 2017. doi:10.1001/jamacardio.2017.0283 eTable 1. Inclusion and Exclusion Criteria eTable 2. CHD Diagnoses With Corresponding ICD-9 Codes eTable 3. Outcomes eTable 4. Medical Comorbidities eTable 5. Type of CHD This supplementary material has been provided by the authors to give readers additional information about their work. © 2017 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 10/01/2021 eTable 1. Inclusion and Exclusion Criteria Inclusion Criteria ICD-9 Codes ICD-9 Procedure Codes DRG Codes 72[0-1], 7221, 7229, 7231, 7239, 724, 72[5- 370, 371, 372, 373, Delivery Admission* V27, 650 9], 7322, 7359, 736, 740, 741, 742, 744, 7499 374, 375 Cesarean Section 669.71 74.0X, 74.1X, 74.2X, 74.4X Exclusion Criteria Ectopic and Molar Pregnancy 63X Dilation and Curettage or Intra- 69.01, 69.51, 74.91, 75.0 amniotic injection for abortion X is any number 0-9. *From Kuklina EV et al. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Matern Child Health J. 2008. © 2017 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 10/01/2021 eTable 2. CHD Diagnoses With Corresponding ICD-9 Codes Complex Congenital Heart Defects ICD-9 Codes Endocardial Cushion Defects 745.6 Hypoplastic Left Heart Syndrome 746.7 Tetralogy -

Reoperation for Tricuspid Regurgitation After Total Correction of Tetralogy of Fallot
Original Reoperation for Tricuspid Regurgitation after Total Article Correction of Tetralogy of Fallot Yoshikazu Hachiro, MD, Nobuyuki Takagi, MD, Tetsuya Koyanagi, MD, and Tomio Abe, MD Background: The aim of this study is to review the outcome of reoperation for severe tricus- pid regurgitation after repair of tetralogy of Fallot . Methods: Between 1972 and 2000, 12 patients underwent reoperation on the tricuspid valve after total correction of tetralogy of Fallot. The mean age at the time of reoperation was 17 years (range, 1 to 39 years). The mean interval between the initial correction and the reoperation was 7.8 years (range, 10 days to 19 years). The functional class was New York Heart Association class II in 2 patients and class III or IV in 10. Six patients underwent tricuspid valve repair, and the others underwent tricuspid valve replacement. Results: Hospital mortality was 16.7% (2/12). Three patients (30%, 3/10) required a second reoperation 1.6, 9.2, and 15.6 years after the most recent reoperation with no deaths. The rea- sons for second reoperation were failure of the tricuspid valve repair in two and a thrombosed valve in one. There were two late deaths. Mean overall event-free actuarial survival at 10 years was 46.3%. Conclusion: Reoperation for severe tricuspid regurgitation after total correction of tetralo- gy of Fallot was associated with a high operative mortality and disappointing long-term results. Tricuspid regurgitation after corrective surgery for tetralogy of Fallot must be diag- nosed promptly and cured, as tolerance is poor because of postoperative right ventricular insufficiency. -
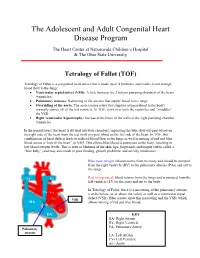
Tetralogy of Fallot (TOF)
The Adolescent and Adult Congenital Heart Disease Program The Heart Center at Nationwide Children’s Hospital & The Ohio State University Tetralogy of Fallot (TOF) Tetralogy of Fallot is a congenital heart defect that is made up of 4 problems and results in not enough blood flow to the lungs: • Ventricular septal defect (VSD): A hole between the 2 bottom pumping chambers of the heart (ventricles) • Pulmonary stenosis: Narrowing of the arteries that supply blood to the lungs • Overriding of the aorta: The aorta (major artery that supplies oxygen blood to the body) normally comes off of the left ventricle. In TOF, it sits over both the ventricles and “straddles” the VSD. • Right ventricular hypertrophy: Increased thickness of the walls of the right pumping chamber (ventricle) In the normal heart, the heart is divided into four chambers, separating the blue (low-oxygen) blood on the right side of the heart from the red (with oxygen) blood on the left side of the heart. In TOF, this combination of heart defects leads to reduced blood flow to the lungs as well as mixing of red and blue blood across a “hole in the heart” or VSD. This allows blue blood to pump out to the body, resulting in low blood oxygen levels. This is seen as blueness of the skin, lips, fingernails and tongue (often called a “blue baby”) and may also result in poor feeding, growth problems, and activity intolerance. Blue (low-oxygen) blood returns from the body and should be pumped from the right ventricle (RV) to the pulmonary arteries (PAs) and out to Aorta the lungs. -

Congenital Cardiac Surgery ICD9 to ICD10 Crosswalks Page 1 of 4 8
Congenital Cardiac Surgery ICD9 to ICD10 Crosswalks ICD-9 code ICD-9 Descriptor ICD-10 Code ICD-10 Descriptor 164.1 Malignant neoplasm of heart C38.0 Malignant neoplasm of heart 164.1 Malignant neoplasm of heart C45.2 Mesothelioma of pericardium 212.7 Benign neoplasm of heart D15.1 Benign neoplasm of heart 425.11 Hypertrophic obstructive cardiomyopathy I42.1 Obstructive hypertrophic cardiomyopathy 425.18 Other hypertrophic cardiomyopathy I42.2 Other hypertrophic cardiomyopathy 425.3 Endocardial fibroelastosis I42.4 Endocardial fibroelastosis 425.4 Other primary cardiomyopathies I42.0 Dilated cardiomyopathy 425.4 Other primary cardiomyopathies I42.5 Other restrictive cardiomyopathy 425.4 Other primary cardiomyopathies I42.8 Other cardiomyopathies 425.4 Other primary cardiomyopathies I42.9 Cardiomyopathy, unspecified 426.9 Conduction disorder, unspecified I45.9 Conduction disorder, unspecified 745.0 Common truncus Q20.0 Common arterial trunk 745.10 Complete transposition of great vessels Q20.3 Discordant ventriculoarterial connection 745.11 Double outlet right ventricle Q20.1 Double outlet right ventricle 745.12 Corrected transposition of great vessels Q20.5 Discordant atrioventricular connection 745.19 Other transposition of great vessels Q20.2 Double outlet left ventricle 745.19 Other transposition of great vessels Q20.3 Discordant ventriculoarterial connection 745.19 Other transposition of great vessels Q20.8 Other congenital malformations of cardiac chambers and connections 745.2 Tetralogy of fallot Q21.3 Tetralogy of Fallot 745.3 Common -
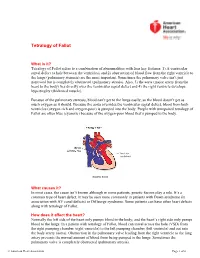
Before Reading the Specific Defect
Tetralogy of Fallot What is it? Tetralogy of Fallot refers to a combination of abnormalities with four key features: 1) A ventricular septal defect (a hole between the ventricles) and 2) obstruction of blood flow from the right ventricle to the lungs (pulmonary stenosis) are the most important. Sometimes the pulmonary valve isn’t just narrowed but is completely obstructed (pulmonary atresia). Also, 3) the aorta (major artery from the heart to the body) lies directly over the ventricular septal defect and 4) the right ventricle develops hypertrophy (thickened muscle). Because of the pulmonary stenosis, blood can’t get to the lungs easily, so the blood doesn’t get as much oxygen as it should. Because the aorta overrides the ventricular septal defect, blood from both ventricles (oxygen-rich and oxygen-poor) is pumped into the body. People with unrepaired tetralogy of Fallot are often blue (cyanotic) because of the oxygen-poor blood that’s pumped to the body. What causes it? In most cases, the cause isn’t known although in some patients, genetic factors play a role. It’s a common type of heart defect. It may be seen more commonly in patients with Down syndrome (in association with AV canal defects) or DiGeorge syndrome. Some patients can have other heart defects along with tetralogy of Fallot. How does it affect the heart? Normally the left side of the heart only pumps blood to the body, and the heart’s right side only pumps blood to the lungs. In a patient with tetralogy of Fallot, blood can travel across the hole (VSD) from the right pumping chamber (right ventricle) to the left pumping chamber (left ventricle) and out into the body artery (aorta). -
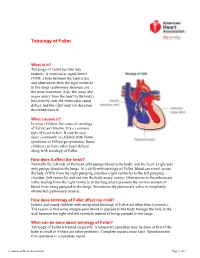
Tetralogy of Fallot
Tetralogy of Fallot What is it? Tetralogy of Fallot has four key features. A ventricular septal defect (VSD; a hole between the ventricles) and obstruction from the right ventricle to the lungs (pulmonary stenosis) are the most important. Also, the aorta (the major artery from the heart to the body) lies directly over the ventricular septal defect, and the right ventricle develops thickened muscle. What causes it? In most children, the cause of tetralogy of Fallot isn’t known. It’s a common type of heart defect. It may be seen more commonly in children with Down syndrome or DiGeorge syndrome. Some children can have other heart defects along with tetralogy of Fallot. How does it affect the heart? Normally the left side of the heart only pumps blood to the body, and the heart’s right side only pumps blood to the lungs. In a child with tetralogy of Fallot, blood can travel across the hole (VSD) from the right pumping chamber (right ventricle) to the left pumping chamber (left ventricle) and out into the body artery (aorta). Obstruction in the pulmonary valve leading from the right ventricle to the lung artery prevents the normal amount of blood from being pumped to the lungs. Sometimes the pulmonary valve is completely obstructed (pulmonary atresia). How does tetralogy of Fallot affect my child? Infants and young children with unrepaired tetralogy of Fallot are often blue (cyanotic). The reason is that some oxygen-poor blood is pumped to the body through the hole in the wall between the right and left ventricle instead of being pumped to the lungs. -

Selected Terms Used in Adult Congenital Heart Disease Jack M
SELECTED TERMS USED IN ADULT CONGENITAL HEART DISEASE JACK M. COLMAN | ERWIN NOTKER OECHSLIN | MATTHIAS GREUTMANN | DANIEL TOBLER ambiguus A With reference to cardiac situs, neither right nor left sided aberrant innominate artery (indeterminate). Latin spelling is generally used for situs ambig- A rare abnormality associated with right aortic arch compris- uus. Syn: ambiguous sidedness. See also situs. ing a sequence of arteries arising from the aortic arch—right carotid artery, right subclavian artery, and then (left) innomi- Amplatzer device nate artery—with the last passing behind the esophagus. This A group of self-centering devices delivered percutaneously by is in contrast to the general rule that the first arch artery gives catheter for closure of abnormal intracardiac and vascular con- rise to the carotid artery contralateral to the side of the aortic nections such as secundum atrial septal defect, patent foramen arch (ie, right carotid artery in left aortic arch and left carotid ovale or patent ductus arteriosus. artery in right aortic arch). Syn: retroesophageal innominate artery. Anderson-Fabry disease See Fabry disease aberrant subclavian artery Right subclavian artery arising from the aorta distal to the left aneurysm of sinus of Valsalva subclavian artery. Left aortic arch with (retroesophageal) aber- See sinus of Valsalva/aneurysm. rant right subclavian artery is the most common aortic arch anomaly. It was first described in 1735 by Hunauld and occurs anomalous pulmonary venous connection in 0.5% of the general population. Syn: lusorian artery. See also Pulmonary venous connection to the right side of the heart, vascular ring. which may be total or partial. -

Having an Echocardiogram to Screen for a Bicuspid Aortic Valve
Having an echocardiogram to screen for a bicuspid aortic valve UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm What is a bicuspid aortic valve? The aortic valve sits between the main chamber of the heart (the left ventricle) and the aorta. Its function is to ensure that blood flows correctly forward from the left ventricle into the aorta. Normally it has three thin leaflets which open as the heart contracts and then close to prevent back-flow of blood towards the ventricle. Aortic valve Tricuspid aortic valve (normal) Bicuspid aortic valve (abnormal) Some people may be born with an aortic valve made up of only 2 leaflets. The valve is then called bicuspid. This may cause problems with the functioning of the valve in that it may be more prone to gradually becoming narrowed or leaky. Sometimes a bicuspid valve may be associated with widening of the portion of the aorta that is connected to it. Widening of the aorta may occur in some relatives even if they have an aortic valve with 3 leaflets. If this widening is significant it is known as an aortic aneurysm. Is a bicuspid valve a common problem? Studies have suggested that this is not an uncommon valve problem and may occur in up to 1 in 200 people. 2 | PI18_1443_03 Having an echocardiogram to screen for a bicuspid aortic valve Recently there has been evidence that this condition may be genetic and thus have a tendency to run in a family. -

Echo in Asymptomatic Mitral and Aortic Regurgitation
2017 ASE Florida | Orlando, FL October 9, 2017 | 10:40 – 11:00 PM | 20 min | Grand Harbor Ballroom South Echo in Asymptomatic Mitral and Aortic Regurgitation Muhamed Sarić MD, PhD, MPA Director of Noninvasive Cardiology | Echo Lab Associate Professor of Medicine Disclosures Speakers Bureau (Philips, Medtronic) Advisory Board (Siemens) Regurgitation Axioms ▪Typically, regurgitation is NOT symptomatic unless severe ▪The opposite is not true: Severe regurgitation may be asymptomatic ▪ Chronic regurgitation leads to chamber dilatation on either side of the regurgitant valve Regurgitation Discovery ▪ Regurgitation as a anatomic entity was recognized in the 17th century ▪ Regurgitation was first clinically diagnosed by auscultation in the 19th century, well before the advent of echocardiography First Use of Regurgitation Term in English 1683 W. Charleton Three Anat. Lect. i. 18 Those [valves] that are placed in the inlet and outlet of the left Ventricle, to obviate the regurgitation of the bloud into the arteria venosa, and out of the aorta into the left Ventricle. Walter Charleton (1619 – 1707) English Physician Heart Murmur OXFORD ENGLISH DICTIONARY DEFINITION ▪ Any of various auscultatory sounds ▪ Adventitious sounds of cardiac or vascular origin [that is, separate from standard heart sounds: S1, S2, S3, S4] ▪ Sometimes of no significance ▪ But sometimes caused by valvular lesions of the heart or other diseases of the Στῆθος : Stēthos = chest circulatory system René Laënnec Stethoscope (1781 – 1826) (‘Chest examiner’) French Physician Hollow wooden cylinder Inventor of stethoscope in 1816 Laënnec Performing Auscultation Painted by Robert Alan Thom (1915 – 1979), American illustrator Commissioned by Parke, Davis & Co. 1816 1832 René Laënnec, James Hope French physician British physician Invents MONAURAL stethoscope separates MS from MR murmur 1852 1862 George Cammann Austin Flint Sr.