Laboratory Experiences in Glasses and Traditional Ceramics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The New York State College of Ceramics
ALFRED UNIVERSITY PUBLICATION The New York State College of Ceramics Catalogue Number for 1939-40 Announcemel1ts for 1940-41 Vol. XV November, 1939 No.9 Published ten limes a year by AlitOed University: Monthly in January, April, l,me, October} NovemberJ December, and semi-monthly in February and March. Entered as second class matter at Alfred, N. Y., Janl/ary 25, 1902, Imder Act of Congress July 16, 1894. Acceptance for mailing at special rate of postage provided tor in Section 1103, Act of Octobet' 3, 1917/ authorized 011 Jflly 3, 1918. fALFRED UNIVERSITY PUBLICATION Vol. xv Novembct, 1939 No.9 TIle Nevv York: State College of Ceralnics Catalogue N umber for 1939-40 Announcements for 1940·-41 AI,FRED, NEW YORK TABLE OF CONTENTS L Calendar ........................................ ...... , .......... 5,6 2, Personnel of the Administration and Faculty. .. ..................... 7 3. History, Objectives and Policies....... .. .. ,......................... 9 4. General Information ............ ,....... ........ ,.... .... , .. :.... 14 5. Adluission ........................... ,.. ..... :.............. 24 6. Expenses .................... .. .......... .. 28 7. Scholastic Requirements .......... .................................. 31 8. Departments of Instruction ................... .. 33 Ceramic Engineering ........... .............. .................... 33 GJass TechnoJogy ................ , ........... .. 35 Industrial CCHunic Design ....................... .................. 36 I~esearch .. .. ................ 38 9. Description of Courses -

Ceramic Engineering Building
CERAMIC ENGINEERING BUILDING UNIVERSITY OF ILLINOIS URBANA CHAMPAIGN, ILLINOIS Description of the Building and Program of Dedication, December 6 unci 7, 1916 THE TRUSTEES THE PRESIDENT AND THE FACULTY OF THIS UNIVERSITY OF ILLINOIS CORDIALLY INVITE YOU TO ATTEND THE DEDICATION OF THE CERAMIC ENGINEERING BUDUDING ON WEDNESDAY AND THURSDAY DECEMBER SIXTH AND SEVENTH NINETEEN HUNDRED SIXTEEN URBANA. ILLINOIS CERAMIC ENGINEERING BUILDING UNIVERSITY OF ILLINOIS URBANA - - CHAMPAIGN ILLINOIS DESCRIPTION OF BUILDING AND PROGRAM OF DEDICATION DECEMBER 6 AND 7, 1916 PROGRAM FOR THE DEDICATION OP THE CERAMIC ENGINEERING BUILDING OF THE UNIVERSITY OF ILLINOIS December 6 and 7> 1916 WEDNESDAY, DECEMBER 6 1.30 p. M. In the office of the Department of Ceramic Engineering, Room 203 Ceramic Engineering Building Meeting of the Advisory Board of the Department of Ceramic Engineering: F. W. BUTTERWORTH, Chairman, Danville A. W. GATES Monmouth W. D. GATES Chicago J. W. STIPES Champaign EBEN RODGERS Alton 2.30-4.30 p, M. At the Ceramic Engineering Building Opportunity will be given to all friends of the University to inspect the new building and its laboratories. INTRODUCTORY SESSION 8 P.M. At the University Auditorium DR. EDMUND J. JAMBS, President of the University, presiding. Brief Organ Recital: Guilnant, Grand Chorus in D Lemare, Andantino in D-Flat Faulkes, Nocturne in A-Flat Erb, Triumphal March in D-Flat J. LAWRENCE ERB, Director of the Uni versity School of Music and University Organist. PROGRAM —CONTINUED Address: The Ceramic Resources of America. DR. S. W. STRATTON, Director of the Na tional Bureau of Standards, Washington, D. C. I Address: Science as an Agency in the Develop ment of the Portland Cement Industries, MR. -

UMR MSE News
September 2005 Vol. 1, No. 1 UMR MSE News A Newsletter for the Alumni of the Metallurgical, Ceramic, and Materials Engineering programs of the Missouri School of Mines/University of Missouri-Rolla Chairman’s Letter here beyond what it has been in many years, and that is Greetings from Rolla! You are now holding the first beginning to garner some widespread attention. For edition of our MSE Newsletter a new venture from the example, a 2004 survey of MSE departments by the faculty, students, and staff of the Metallurgical University Materials Council indicates that the UMR Engineering and Ceramic Engineering programs at the MSE undergrad program is one of the five largest in the University of Missouri-Rolla. Since July 2004, these US. The 2004 US News & World Report listed our pro- st two venerable programs have formed the core of gram as the 41 ranked graduate MSE program in the UMR’s new Materials Science and Engineering US the first time that UMR has been included in these Department. This newsletter will review some of the rankings. highlights of our first year and give you an idea about some plans for the future. We had several changes in departmental personnel this year. Associate Prof. Chris Ramsay, a fixture in the It is worth noting at the outset that the formation of the metallurgical engineering program since 1989, took a MSE department does not signal the end of independ- leave of absence last May to develop his consulting ent ceramic engineering and metallurgical engineering business. Priscilla Winner, the Metallurgy and MSE programs. -

COURSE: EMA 4645, Section 061B Title: Processing of Ceramic Materials Fall 2016
Version 1; July 29, 2016 Syllabus COURSE: EMA 4645, Section 061B Title: Processing of Ceramic Materials Fall 2016 1. Catalog Description – Credits: 3. Introduction to the technology and science of processing ceramic materials, including traditional clay based ceramics, modern technical ceramics, and glasses. Topics include the nature of fine particles, forming methods and consolidation by heat. 2. Prerequisites - EMA 4144 3. Course Objectives - At the end of this course students will be able to understand and apply the basic principles of ceramic processing, including characterization techniques, colloid and surface science, sol-gel techniques, particle mechanics, ceramic forming and sintering. 4. Contribution of course to meeting the professional component - This is a 3 credit course. It provides 3 credits towards engineering sciences. 5. Relationship of course to program outcomes - This course addresses the following MSE Program outcomes: 1 Ability to apply knowledge of mathematics, science, and engineering to materials systems. (High Coverage) Assessment is done of the homework (use of software, spreadsheet calculations) and the three exams. 4 Ability to apply and integrate knowledge of structure, properties, processing, and performance to solve materials selection and design problems within realistic constraints. (Medium coverage) Student’s ability is measured in the exams. Students have to decide what processes they choose for what type of ceramic shape and material. They need to suggest alternative processes and materials. 7 Ability to identify, formulate, and solve engineering problems. (Medium coverage) Questions in the exams address industrial problems that have occurred. These problems are described with everyday language that the students need to translate into engineering terminology to identify the potential engineering problems, formulate the problems and offer several solution pathways. -

Ceramic Materials
Material Science I Ceramic Materials F. Filser & L.J. Gauckler ETH-Zürich, Departement Materials [email protected] HS 2007 Ceramics: Introduction 1 Material Science I Persons in Charge of this Lecture • Dr. F. Filser, HCI G 529, phone 26435, [email protected] • Prof. Dr. L.J. Gauckler HCI G 535, phone 25646, [email protected] • F. Krauss HCI G 538, phone 3 68 34, [email protected] • Dipl.-Ing. J. Kübler EMPA Dübendorf, phone 044 823 4223, [email protected] Ceramics: Introduction 2 Material Science I Overview & preliminary schedule (HS 2007) Nov 26, 07 Introduction on ceramic materials, technology, applications Dec 03, 07 Crystal structures of ceramic materials Dec 10, 07 Potential well of bonding and physical properties & Examples of Structural ceramic materials Dec 17, 07 Examples of structural ceramic materials Dec 21, 07 term finish Ceramics: Introduction 3 Material Science I Overview & preliminary schedule (FS 2008) Feb 18, 08 term starts (5 x ceramic & 9 x polymer) Feb 19, 08 Glass Feb 26, 08 Toughness (JK) Mar 04, 08 Strength & Weibull statistics (JK) Mar 11, 08 Subcritical crack growth, SPT-Diagrams (JK) Mar 18, 08 Proof-testing, creep, thermical properties (JK) Apr 01, 08 polymer part (Prof. D. Schlüter) May 30, 08 term finish Ceramics: Introduction 4 Material Science I Documentation Visit our homepage @ http://ceramics.ethz.ch -> education -> courses -> Materialwissenschaft I und II Ceramics: Introduction 5 Material Science I Sources of Information - ETH Bib -NEBIS http://www.ethbib.ethz.ch/ http://www.nebis.ch/ Ceramics: Introduction 6 Material Science I Recommended Reading • Askeland & Phulé: Science and Engineering of Materials, 2003 • Barsoum MW: Fundamentals of Ceramics. -

College of Engineering MSE Materials Science and Engineering
College of Engineering MSE Materials Science and Engineering MSE 101 MATERIALS ENGINEERING. (1) An introduction to the materials engineering profession. Professional growth, conduct, ethics and organizations. Introduction to the techniques of materials engineering. MSE 201 MATERIALS SCIENCE. (3) Microscopic and macroscopic structure as related to the properties of materials with engineering applications. Prereq or concur: MA 114 and freshman chemistry. MSE 202 MATERIALS SCIENCE LABORATORY. (1) To teach students the basic materials characterization laboratory techniques and demonstrate the difference in properties between different types of materials. Prereq: Concurrent enrollment in MSE 201. MSE 212 ELECTRONIC PROPERTIES OF MATERIALS. (3) Modern ideas on the engineering properties of solids, crystallographic properties; relationship of properties to structure and electronic properties of materials. Prereq: PHY 232 and 242, MA 214 concurrent. MSE 301 MATERIALS SCIENCE II. (3) Introduction to processing of ceramic, polymer and composite materials; relating the structure and bonding in these materials to their properties; considerations in choosing appropriate materials for engineering applications. Prereq: MSE 201, or consent of instructor. *MSE 351 MATERIALS THERMODYNAMICS. (3) Solution thermodynamics; partial molal quantities; ideal and non-ideal solutions; application of thermodynamics to phase equilibria; heterogeneous equilibria; free energy-composition relationships; temperature-pressure relationships. Prereq: MSE 201, MA 213 or concurrent. MSE 395 INDEPENDENT WORK IN MATERIALS ENGINEERING. (1-3) Research for undergraduate departmental students. May be repeated to a maximum of 12 credits. Prereq: Department major and approval of chairperson. MSE 401G METAL AND ALLOYS. (3) Crystal structures, phase diagrams, diffusion, nucleation and growth, deformation, recovery, recrystallization and grain growth are discussed to understand the structure-property relations in metals and alloys. -

Ceramic Engineering 1
Ceramic Engineering 1 CERAMIC ENGINEERING The ceramic engineering program in the department of materials CER ENG 5000 Special Problems (IND 0.0-6.0) science and engineering offers comprehensive graduate education in Problems or readings on specific subjects or projects in the department. a number of areas including structural ceramics, electronic materials, Consent of instructor required. high temperature materials, and glass. Further information on these opportunities and facilities available to carry out research in ceramic CER ENG 5001 Special Topics (LEC 0.0-6.0) engineering may be found under materials science and engineering. This course is designed to give the department an opportunity to test a Degree Requirements new course. Variable title. M.S. and Ph.D. degrees are offered in ceramic engineering. The total CER ENG 5002 Cooperative Training (IND 1.0-3.0) number of hours required for the M.S. in ceramic engineering is 30. A On-the-job experience gained through cooperative education with minimum of 6 hours of 6000-level lectures and a minimum of 11 hours of industry, with credit arranged through departmental cooperative graduate research on the Missouri S&T campus are required. A maximum advisor. Grade received depends on quality of reports submitted at work of 6 hours of 4000-level lecture credit may be accepted. supervisor's evaluation. The minimum number of hours (beyond the bachelor's degree) required for the Ph.D. in ceramic engineering is 72. At least 12 hours of course CER ENG 5040 Oral Examination (IND 0.0) work outside of ceramic engineering is recommended, a minimum of 24 After completion of all other program requirements, oral examinations for hours will be dissertation research, and a minimum of 24 hours must be on-campus M.S./Ph.D. -
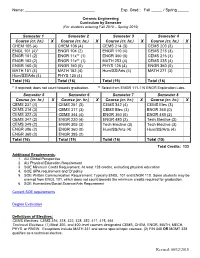
Ceramic Engineering Curriculum by Semester (For Students Entering Fall 2018 – Spring 2019)
Name: ________________________________ Exp. Grad.: Fall _____ / Spring _____ Ceramic Engineering Curriculum by Semester (For students entering Fall 2018 – Spring 2019) Semester 1 Semester 2 Semester 3 Semester 4 Course (cr. hr.) X Course (cr. hr.) X Course (cr. hr.) X Course (cr. hr.) X CHEM 105 (4) CHEM 106 (4) CEMS 214 (3) CEMS 203 (3) ENGL 101 (4)* ENGR 104 (2) ENGR 110 (4) CEMS 215 (3) ENGR 101 (2) ENGR 11x** (1) ENGR 360 (0) CEMS 216 (3) ENGR 102 (2) ENGR 11x** (1) MATH 253 (4) CEMS 235 (4) ENGR 160 (0) ENGR 160 (0) PHYS 126 (4) ENGR 360 (0) MATH 151 (4) MATH 152 (4) Hum/SS/Arts (4) MATH 271 (3) Hum/SS/Arts (4) PHYS 125 (4) Total (16) Total (16) Total (19) Total (16) * If required; does not count towards graduation. ** Select from ENGR 111-116 ENGR Exploration Labs. Semester 5 Semester 6 Semester 7 Semester 8 Course (cr. hr.) X Course (cr. hr.) X Course (cr. hr.) X Course (cr. hr.) X CEMS 237 (4) CEMS 251 (3) CEMS 342 (4) CEMS Elec (3) CEMS 314 (3) CEMS 317 (3) CEMS Elec (3) ENGR 360 (0) CEMS 322 (3) CEMS 344 (4) ENGR 360 (0) ENGR 480 (2) CEMS 347 (2) ENGR 220 (4) ENGR 480 (2) Tech Elective (3) CEMS 349 (2) ENGR 305 (3) Tech Elective (3) Tech Elective (3) ENGR 306 (2) ENGR 360 (0) Hum/SS/Arts (4) Hum/SS/Arts (4) ENGR 360 (0) ENGR 395 (2) Total (16) Total (19) Total (16) Total (15) Total Credits: 133 Additional Requirements: 1. -

History of the Department of Ceramic Engineering the Department Was Founded Largely Due to the Efforts of Edward Orton, Jr
History of the Department of Ceramic Engineering The department was founded largely due to the efforts of Edward Orton, Jr. who was the son of Dr. Edward Orton and Mary Jennings Orton and was born at Chester, N.Y., October 8, 1863. He was taken to Yellow Springs, Ohio, when his father became principal of the preparatory school of Antioch College in 1865. He doubtless learned geology at his father's knee, as "Dr. Orton's geological work at Antioch received official recognition in 1869 when he became one of the two principal assistants to the State Geologist of Ohio, Dr. J.S. Newberry." As a lad of nine years of age, he saw his father become President of Antioch College and a year later inducted as the first President of the newly established Ohio State Agricultural and Mechanical College, as well as its professor of geology, mining and metallurgy. It will thus be seen that geologic science and admin- istrative art entered early into his life. Boyhood and College Days From 1873 to 1877, he attended the public schools of the city of Columbus. At fourteen years of age he entered the Preparatory Depart- ment of the Ohio State Agricultural and Mechanical College, being the 238th matriculant, and was transferred to the Collegiate Department in 1881. Living with his father in the President's residence at the Fifteenth Avenue entrance to the campus, he had exceptional oppor- tunities to see the normal life of a gentleman, an educator, a scie~tist, and an administrator, and to meet many prominent men in those wa:~s of life. -

1945-46 Ceramics Catalogue.Pdf
,~-,-~, - . " _ .-4_ _~ THE NEW YORK STATE COLLEGE OF CERAMICS Catalogue Number for 1945-1946 A n,nouncements for 1946-47 ALFRED UNIVERSITY PUBLICATION Vol. XXI November, 1945 No.9 Published len times a year by Alfred UniversilY: Monthly in /anualY, April, ]sme, OClober, November, December, and semi-monthly in Fehruary and March. Bnlered as secona class mailer aI Alfred, N. Y., January 25, 1902, flnde, Acl of Congress /II/y 16, 1894. Aueplance for mailing at special rale of postage provided for in S8ttion 1103. Act of October 3, 1917. allihorized on July 3, 1918. TABLE OF CONTENTS PAGE 1. Calendar .. ,............................... ........................ 4, 5 2. Personnel of the Administration and Faculty. .. ................•.. 6. 7 3. History, Objectives and Policies. .. 9 4. General Information ..................................................• 14 5. Admission ...........................................................• 22 6. Scholastic Regulations ... .. 26 7. Expenses ........ , . .. .. .. .. .. .. .. .. .. .. 30 8. Departments of Instruction ........................... , . 34 Ceramic Engineering ..............................................•.. 34 General Ceramic Technology ................................. " . .. .. 38 Glass Technology . • . • . 36 Industrial Ceramic Design. 39 Research ..........................................................• 41 9. Descriptions of Courses. .. 43 Ceramic Engineering ................................................. 43 Glass Technology . 45 Industrial Ceramic Design. 47 Chemistry .......... -

Ultra-High Temperature Ceramic Materials for Extreme Environment Applications by E
UHTCs: Ultra-High Temperature Ceramic Materials for Extreme Environment Applications by E. Wuchina, E. Opila, M. Opeka, W. Fahrenholtz, and I. Talmy .That’s not just hot SiO(g) instead of a protective SiO2 layer) 3000°C..– it’s EXTREMELY can occur at very high temperatures hot. It is above the melting or (> 1350°C, depending on PO2) and decomposition temperatures for most reduced system pressures. In addition, of the materials known to man. But decomposition of already-formed SiO2, in the world of extreme environment or the interface reaction between SiC engineering, it is just a baseline. The list and SiO2 results in SiO(g) formation at of materials with melting temperatures high temperatures and reduced pressure above 3000°C is limited to perhaps 15 environments. Other materials, such as elements or compounds (Table I). That TiB2, TiC, NbB2, NbC, while having high is a pretty small palette of materials to melting temperatures, form oxides with draw from for an engineer or designer. low melting points (TiO2 – Tm = 1840°C and Nb2O5 – Tm = 1485°C). Graphite has Table I. Materials with melting temperatures the highest melting temperature of any above 3000°C. material known, but starts to burn Carbon TaB Re thet 800°C. While it is a most widely 2 used material in high-temperature W HfC BN applications, it must be protected by HfB HfN ZrC coatings for long-term use. 2 What we are left with are the borides, TaC ZrB2 TiC carbides, and nitrides of the Group IV & V elements, as well as mixtures based on TaN NbC ThO 2 these compounds. -

2016-2017 Ceramic Engineering Curriculum
Name: Key: Done In Progress Possible based on prerequisites 2016-2017 Ceramic Engineering Curriculum This chart was prepared by Freshman Engineering using the 2016-2017 catalog. It is designed to assist in advising and course selection; refer to the student's catalog requirement year for official requirements and to the student's degree audit for official progress. FEP Math 1103 Fundamentals of Algebra Prerequisite: Entrance requirements. 3 FEP Math 1120 College Algebra Prerequisite: By placement examination. 5 FEP Math 1140 College Algebra Prerequisite: By placement examination. 3 FEP Math 1160 Trigonometry Prerequisite: Math 1120 or 1140 with a grade 2 of "C" or better; or by placement exam. Prerequisites FEP Chem 1100 Introduction to Laboratory Safety & Hazardous Materials 1 FEP Fr Eng 1100 Study & Careers in Engineering 1 FEP Chem 1310 General Chemistry I Prerequisite: Entrance requirements. 4 FEP Chem 1319 General Chemistry Laboratory Prerequisite: Preceded or accompanied by both 1 Chem 1310 and Chem 1100. Math 1214 Calculus for Engineers I Prerequisites: A grade of "C" or better in both 4 Math 1160 and one of Math 1120 or Math 1140; or by placement exam. Semester 1 Semester FEP Hum/Soc Sci English 1120 Exposition and Argumentation 3 Requirement- English FEP Hum/Soc Sci History/Pol one of 1. History 1200 Modern Western Civilization 3 Elective - Sci these 2. History 1300 American History to 1877 History 3. History 1310 American History Since 1877 4. Pol Sci 1200 American Government 16 Chemistry Various 1. Met Eng 1210 Chemistry of Materials (preferred) 1. Prerequisite: "C" or better grade in Chem 3 Elective 2.