VAPOR-LIQUID EQUILIBRIUM DATA COLLECTION Chemistry Data Series
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
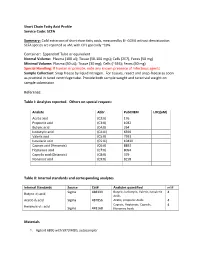
Short Chain Fatty Acid Profile Service Code: SCFA
Short Chain Fatty Acid Profile Service Code: SCFA Summary: Cold extraction of short chain fatty acids, measured by EI- GCMS without derivatization. SCFA species are reported as uM, with CV's generally ~10%. Container: Eppendorf Tube or equivalent Normal Volume: Plasma (100 ul); Tissue (50-100 mgs); Cells (2E7), Feces (50 mg) Minimal Volume: Plasma (50 uL); Tissue (30 mg); Cells (~5E6); Feces (40 mg) Special Handling: If human or primate, note any known presence of infectious agents. Sample Collection: Snap freeze by liquid nitrogen. For tissues, resect and snap-freeze as soon as practical in tared centrifuge tube. Provide both sample weight and tared vial weight on sample submission Reference: Table I: Analytes reported. Others on special request: Analyte Abbr. PubCHEM LOQ(uM) Acetic acid (C2:0) 176 Propionic acid (C3:0) 1032 Butyric acid (C4:0) 264 Isobutyric acid (C4:0i) 6590 Valeric acid (C5:0) 7991 Isovaleric acid (C5:0i) 10430 Caproic acid (Hexanoic) (C6:0) 8892 Heptanoic acid (C7:0) 8094 Caprylic acid (Octanoic) (C8:0) 379 Nonanoic acid (C9:0) 8158 Table II: Internal standards and corresponding analytes Internal Standards Source Cat# Analytes quantified mM Sigma 488399 Butyric, Isobutyric, Valeric, Isovaleric 4 Butyric-d7 acid Acids Acetic-d3 acid Sigma 487856 Acetic, propionic Acids 4 Caproic, Heptanoic, Caprylic, 4 Hexanoic-d11 acid Sigma 448168 Nonanoic Acids Materials 1. Agilent 6890 with 5973 MSD, autosampler 2. Vortexer 3. Refrigerated centrifuge, capable of 13,000g with eppendorf tube compatible rotor 4. ice bucket, ice 5. Balance 6. Prepared stock solutions of short chain fatty acid standards and isotope-labeled short chain fatty acid internal standards. -

Federal Register/Vol. 81, No. 250/Thursday, December 29, 2016
95886 Federal Register / Vol. 81, No. 250 / Thursday, December 29, 2016 / Rules and Regulations C. Regulatory Flexibility Act (RFA) I. National Technology Transfer and ENVIRONMENTAL PROTECTION Advancement Act (NTTAA) AGENCY This action is not subject to the RFA. This rulemaking does not involve The RFA applies only to rules subject to 40 CFR Part 180 notice-and-comment rulemaking technical standards. requirements under the APA, 5 U.S.C. [EPA–HQ–OPP–2016–0007 and EPA–HQ– J. Executive Order 12898: Federal OPP–2016–0008; FRL–9950–40] 553, or any other statute. This rule is not Actions To Address Environmental subject to notice-and-comment Justice in Minority Populations and Isobutyl Acetate and Isobutyric Acid; requirements because the agency has Low-Income Populations Exemption From the Requirement of a invoked the APA ‘‘good cause’’ The EPA believes that this action is Tolerance exemption under 5 U.S.C. 553(b). not subject to Executive Order 12898 (59 AGENCY: Environmental Protection FR 7629, February 16, 1994) because it D. Unfunded Mandates Reform Act Agency (EPA). (UMRA) does not establish an environmental health or safety standard. This good ACTION: Final rule. This action does not contain any cause final action simply extends the SUMMARY: This regulation establishes unfunded mandate of $100 million or date for the EPA to take action on a more as described in UMRA, 2 U.S.C. exemptions from the requirement of a petition and does not have any impact tolerance for residues of isobutyl acetate 1531–1538, and does not significantly or on human health or the environment. -
![[Agr. Biol. Chem., Vol. 36, No. 1, P. 112---119, 1972] Studies of the Peptide Antibiotic Suzukacillin Part II by Takaaki OOKA An](https://docslib.b-cdn.net/cover/9902/agr-biol-chem-vol-36-no-1-p-112-119-1972-studies-of-the-peptide-antibiotic-suzukacillin-part-ii-by-takaaki-ooka-an-399902.webp)
[Agr. Biol. Chem., Vol. 36, No. 1, P. 112---119, 1972] Studies of the Peptide Antibiotic Suzukacillin Part II by Takaaki OOKA An
[Agr. Biol. Chem., Vol. 36, No. 1, p. 112---119,1972] Studies of the Peptide Antibiotic Suzukacillin Part II By Takaaki OOKA and Isao TAKEDA TechnicalResearch Laboratory, Asahi ChemicalIndustry Co., Ltd., Nakadai-cho3-27, Itabashi-kuTokyo ReceivedJuly 26, 1971 Suzukacillin produced by Trichoderma viride 6301 strain was purified and shown to be separated into two components A and B on thin-layer chromatography. The component A was isolated and crystallized from the mixture of components by alumina column chro matography. The component A is composed of six amino acids, Gly, Glu, Ala, Pro, Val, Leu and an unknown amino acid. This unknown amino acid was identified as a-amino isobutyric acid. It is supposed that a-amino isobutyrie acid is biosynthesized mainly from L-valine by the isotopic experiments. Suzukacillin formation by Trichoderma viride 6301 was stimulated by the addition of L-Asn, GABA, L-Ser, Gly and L-Arg into the medium. Trichoderma viride 6301 strain producing Suzu ments were purchased as reagents from Tokyo Kasei kacillin was isolated by the author's laboratory. Co., Ltd. The following radioactive compounds were This organism accumulated noted amount of surplied by Dai-ichi Chemical Co., Ltd., with the in the peptide antibiotic. It was detected on dicated specific activities (ƒÊCi per mmole): Starch- TLC that the peptide antibiotic Suzukacillin (U)14C (30.8), pyruvic acid-(U)-14C (31.5), glycine- consists of two components A and B. A is (U)14C (45.2), L-alanine-(U)-14C (107), L-glutamic acid- (U)-14C (165), L-proline-(U)-14C (165), L-valine-(U)-14C a major and B is a minor component. -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

Influence of Sargassum Horneri Mitigating Odorous Gas Emissions from Swine Manure Storage Facilities
sustainability Article Influence of Sargassum horneri Mitigating Odorous Gas Emissions from Swine Manure Storage Facilities Lavanya Madhavaraj 1, Ho-Dong Lim 1, Kong-Min Kim 1, Dae-Hyuk Kim 2 and Gui Hwan Han 1,* 1 Center for Industrialization of Agricultural and Livestock Microorganisms (CIALM), 241, Cheomdangwahag-ro, Jeongeup-si 56212, Korea; [email protected] (L.M.); [email protected] (H.-D.L.); [email protected] (K.-M.K.) 2 Department of Molecular Biology, Institute for Molecular Biology and Genetics, Jeonbuk National University, Jeonju 561–756, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-63-536-6713; Fax: +82-63-536-6003 Received: 4 August 2020; Accepted: 11 September 2020; Published: 15 September 2020 Abstract: Manures from livestock industries and farmyards should be managed for land application. Currently, a deep pit or barn system is adopted by many swine farms for manure management, therefore releasing harmful gases and rising the total global emissions of GHGs. This research focuses on the effectiveness of the brown seaweed Sargassum horneri as a masking agent to mitigate odor-generating gaseous pollutants and reduce the emissions of volatile fatty acids (VFAs) from swine manure storage facilities. Using an optimized procedure, we compared the gaseous emissions from two manure storage barns, one containing swine manure masked with S. horneri and the other without masking as a control, over a 30-day period. The results showed that, compared to the control, seaweed masking significantly reduced the sulfide and VFA contents. Furthermore, reductions of 99.48% in H S, 60 5.21% in NH and 74.28 2.14% in gaseous amine emissions were observed 2 ± 3 ± within the experimental period. -

United States Patent Office Patented Apr
3,576,860 United States Patent Office Patented Apr. 27, 1971 2 3,576,860 Further objects, aspects and advantages of the inven PREPARATION OF CHLOROACETYL CHLORIDE . tion will be apparent from the description which follows. Dimitri A. Zazaris, Belleville, Ill., assignor to Chlorination of acetic acid alone in the absence of a Monsanto Company, St. Louis, Mo. catalyst by prior art processes usually requires relatively No Drawing. Filed Dec. 20, 1967, Ser. No. 691,975 5 high temperatures, 110 to 120° C., in order for the re Int, C. C07c53/20 action to occur. Temperature in this range, however, re U.S. C. 260-539 9 Claims Sult in a high yield of dichloroacetic acid. In order to re duce the reaction temperature, acetic anhydride, acetyl chloride, inorganic salts or combination of the three have ABSTRACT OF THE DISCLOSURE O been added to the acetic acid. Reaction ompositions of 85 weight percent acetic anhydride and 15 weight percent Chlorination of acetic anhydride and acetic anhydride acetic acid have been utilized. In the chlorination of this acetic acid mixtures in the presence of lithium chloride to mixture, the product obtained was predominantly mono yield a mixture of monochloroacetyl chloride and mono chloroacetic acid. This is contrary to what would be ex chloroacetic acid having a small concentration of the poly pected since theoretically acetic anhydride should give 1 chlorinated derivatives. mole of acid and 1 mole of acid halide per mole of an hydride. The use of many inorganic salts did not mate rially change this result. Many of the present commercial herbicides, such as the It has previously been determined that the low yield of chloroacetamides and "2,4-D,' utilize a monochloroacetyl 20 monochloroacetyl chloride is due to the interactions of chloride or monochloroacetic acid as an intermediate. -

Safety Data Sheet
SAFETY DATA SHEET Creation Date 15-Feb-2011 Revision Date 10-Dec-2020 Revision Number 7 SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier Product Description: Chloroacetic acid Cat No. : 108510000; 108510010; 108510025; 108510040; 108512500 Synonyms MCA CAS-No 79-11-8 EC-No. 201-178-4 Molecular Formula C2 H3 Cl O2 Reach Registration Number 01-2119459589-18 1.2. Relevant identified uses of the substance or mixture and uses advised against Recommended Use Laboratory chemicals. Sector of use SU3 - Industrial uses: Uses of substances as such or in preparations at industrial sites Product category PC21 - Laboratory chemicals Process categories PROC15 - Use as a laboratory reagent Environmental release category ERC6a - Industrial use resulting in manufacture of another substance (use of intermediates) Uses advised against No Information available 1.3. Details of the supplier of the safety data sheet Company UK entity/business name Fisher Scientific UK Bishop Meadow Road, Loughborough, Leicestershire LE11 5RG, United Kingdom EU entity/business name Acros Organics BVBA Janssen Pharmaceuticalaan 3a 2440 Geel, Belgium E-mail address [email protected] 1.4. Emergency telephone number For information US call: 001-800-ACROS-01 / Europe call: +32 14 57 52 11 Emergency Number US:001-201-796-7100 / Europe: +32 14 57 52 99 CHEMTREC Tel. No.US:001-800-424-9300 / Europe:001-703-527-3887 SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the substance or mixture CLP Classification -

Safety Data Sheet MCAA
SAFETY DATA SHEET MONOCHLOROACETIC ACID 80% - 90% AQ Solution Revision Date: September 2019 Previous date: May 2014 Print Date: September 2019 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1 Product information Commercial Product Name Monochloroacetic Acid Chemical Formula CICH2CO2H Molecular wt. 94.50 80-90% Solution 1.2 Relevant identified uses of the substance or mixture and uses advised against Use of the Substance/Mixture Recommended restrictions on use Reserved for industrial and professional use. 1.3 Details of the supplier of the safety data sheet Niacet Corporation 400 47th Street Niagara Falls, NY 14304 U.S.A. Telephone +1 716-285-1474 Telefax +1 716-285-1497 [email protected] 1.4 Emergency telephone number For Niacet Corporation, Niagara Falls, U.S.A. products: Chemtrec: +1 (800) 424 9300, +1 (703) 527-3887 2. HAZARDS IDENTIFICATION 2.1 Classification of the substance or mixture Classification according to OSHA Hazards Target Organ Effect (Central Nervous System, Heart, Skeletal Muscle, Kidney), Toxic by ingestion, Toxic by skin absorption, Corrosive Rapidly absorbed through skin Classification according to GHS Classification Acute toxicity, Oral (Category 3) Acute toxicity, Inhalation (Category 3) Skin corrosion (Category 1B) Serious eye damage (Category 1) 1/12 SAFETY DATA SHEET MONOCHLOROACETIC ACID 80% - 90% AQ Solution Revision Date: September 2019 Previous date: May 2014 Print Date: September 2019 Acute aquatic toxicity (Category 1) 2.2 Label elements Labeling according to GHS Label elements, including precautionary statements Pictogram Signal word Danger Hazard statement(s) H301 + H311 Toxic if swallowed or in contact with skin H314 Causes severe skin burns and eye damage. -

Chloroacetic Acid ≥99,5 %, P.A
Safety data sheet according to Regulation (EC) No. 1907/2006 (REACH) Chloroacetic acid ≥99,5 %, p.a. article number: 9849 date of compilation: 2016-06-17 Version: 1.1 en Revision: 2021-02-17 Replaces version of: 2016-06-17 Version: (1) SECTION 1: Identification of the substance/mixture and of the company/ undertaking 1.1 Product identifier Identification of the substance Chloroacetic acid ≥99,5 %, p.a. Article number 9849 Registration number (REACH) It is not required to list the identified uses be- cause the substance is not subject to registration according to REACH (< 1 t/a). Index number in CLP Annex VI 607-003-00-1 EC number 201-178-4 CAS number 79-11-8 1.2 Relevant identified uses of the substance or mixture and uses advised against Relevant identified uses: Laboratory chemical Laboratory and analytical use Uses advised against: Do not use for squirting or spraying. Do not use for products which come into direct contact with the skin. Do not use for products which come in- to contact with foodstuffs. Do not use for private purposes (household). 1.3 Details of the supplier of the safety data sheet Carl Roth GmbH + Co KG Schoemperlenstr. 3-5 D-76185 Karlsruhe Germany Telephone:+49 (0) 721 - 56 06 0 Telefax: +49 (0) 721 - 56 06 149 e-mail: [email protected] Website: www.carlroth.de Competent person responsible for the safety data :Department Health, Safety and Environment sheet: e-mail (competent person): [email protected] 1.4 Emergency telephone number Name Street Postal Telephone Website code/city National Poisons Information Beaumont Road Dublin 9 01 809 2166 https:// Centre www.poisons.ie/ Beaumont Hospital Ireland (en) Page 1 / 17 Safety data sheet according to Regulation (EC) No. -

Study on Gas-Phase Mechanism of Chloroacetic Acid Synthesis by Catalysis and Chlorination of Acetic Acid
Asian Journal of Chemistry; Vol. 26, No. 2 (2014), 475-480 http://dx.doi.org/10.14233/ajchem.2014.15484 Study on Gas-Phase Mechanism of Chloroacetic Acid Synthesis by Catalysis and Chlorination of Acetic Acid * JIAN-WEI XUE , JIAN-PENG ZHANG, BO WU, FU-XIANG LI and ZHI-PING LV Research Institute of Special Chemicals, Taiyuan University of Technology, Taiyuan 030024, Shanxi Province, P.R. China *Corresponding author: Fax: +86 351 6111178; Tel: +86 351 60105503; E-mail: [email protected] Received: 14 March 2013; Accepted: 17 May 2013; Published online: 15 January 2014; AJC-14570 The process of acetic acid catalysis and chlorination for synthesizing chloroacetic acid can exist in not only gas phase but also liquid phase. In this paper, the gas-phase reaction mechanism of the synthesis of chloroacetic acid was studied. Due to the high concentration of acetic acid and the better reaction mass transfer in the liquid-phase reaction, the generation amount of the dichloroacetic acid was higher than that in the gas-phase reaction. Under the solution distillation, the concentration of acetyl chloride, whose boiling point is very low, was very high in the gas phase, sometimes even up to 99 %, which would cause the acetyl chloride to escape rapidly with the hydrogen chloride exhaust, so that the reaction slowed down. Therefore, series reactions occured easily in the gas-phase reaction causing the amount of the dichloroacetic acid to increase. Keywords: Gas phase, Catalysis, Chlorination, Chloroacetic acid, Acetic acid. INTRODUCTION Martikainen et al.3 summed up the reaction mechanism that was consistent with a mechanism found by Sioli according Chloroacetic acid is not only a fine chemical product but to the system condition experiment and systematic theoretical also an important intermediate in organic synthesis. -

Extraction of Benzoic Acid Dr
CHEM 2423 Extraction of Benzoic Acid Dr. Pahlavan EXPERIMENT 6 - Extraction Determination of Distribution Coefficient Purpose: a) To purify samples of organic compounds that are solids at room temperature b) To dissociate the impure sample in the minimum amount of an appropriate hot solvent Equipment / Materials: Benzoic acid solution ring& ring stand burette burette clamp spatula 0.1 M NaOH (or 0.02 M) solution separatory funnel methylene dichloride 125 ml Erlemeyer flask 10 and 50 mL graduated cylinders 50 and 100 mL beakers funnel Discussion: Crystallization, purification, and isolation (may only be restricted to a solid) are insufficient ways to separate mixtures of compounds. Extraction is the recovery of a substance from a mixture by bringing it into contact with a solvent, which dissolves the desired material. Partitioning is the separation between two distinct phases (immiscible liquids) and also called fractional separation. Like recrystallization and distillation, extraction is a separation technique frequently employed in the laboratory to isolate one or more components from a mixture. Unlike recrystallization and distillation, it does not yield a pure product; thus, the former techniques may be required to purify a product isolated by extraction. In the technical sense extraction is based on the principle of the equilibrium distribution of a substance (solute) between two immiscible phases, one of which is usually a solvent. The solvent need not be a pure liquid but may be a mixture of several solvents or a solution of some chemical reagent that will react with one or more components of the mixture being extracted to form a new substance soluble in the solution. -

Safety Data Sheet (SDS) OSHA Hazcom Standard 29 CFR 1910.1200(G) and GHS Rev 03
Page 1/12 Safety Data Sheet (SDS) OSHA HazCom Standard 29 CFR 1910.1200(g) and GHS Rev 03. Reviewed on 09/25/18 Issue date 06/11/15 * 1 Identification ꞏ Product identifier ꞏ Trade name: Monochloroacetic Acid Solution ꞏ Relevant identified uses of the substance or mixture and uses advised against No further relevant information available. ꞏ Product description Monochloroacetic Acid Solution ꞏ Details of the supplier of the safety data sheet ꞏ Manufacturer/Supplier: Dermatologic Lab & Supply, Inc. 608 13th Ave. Council Bluffs, IA USA 51501-6401 Voice: (800) 831-6273 or (712) 323-3269 Fax: (800) 320-9612 or (712) 323-1156 www.delasco.com ꞏ Emergency telephone number: Chemtrec 800-424-9300 * 2 Hazard(s) identification ꞏ Classification of the substance or mixture GHS06 Skull and crossbones Acute Tox. 3 H311 Toxic in contact with skin. Acute Tox. 3 H331 Toxic if inhaled. GHS05 Corrosion Skin Corr. 1B H314 Causes severe skin burns and eye damage. Eye Dam. 1 H318 Causes serious eye damage. GHS09 Environment Aquatic Acute 1H400Very toxic to aquatic life. GHS07 Acute Tox. 4 H302Harmful if swallowed. Label elements ꞏ 41.0 Page 2/12 Safety Data Sheet (SDS) OSHA HazCom Standard 29 CFR 1910.1200(g) and GHS Rev 03. Issue date 06/11/15 Reviewed on 09/25/18 Trade name: Monochloroacetic Acid Solution ꞏ GHS label elements The product is classified and labeled according to the Globally Harmonized System (GHS). ꞏ Hazard pictograms GHS05 GHS06 GHS09 ꞏ Signal word Danger ꞏ Hazard-determining components of labeling: Chloroacetic acid ꞏ Hazard statements Harmful if swallowed.