Slr2 Displacements and Reductive Coupling of Ketones with Olefins in N,N-Diethylacetamide and N-Ethylpyrrolidone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SUMMARY of an INVESTIGATION of the COMPOSITION of a MIDCONTINENT PETROLEUM DISTILLATE, BOILING BETWEEN 100° and 130° C.L
U. S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS RESEARCH PAPER RP1174 Part of Journal of Research of the National Bureau of Standards, Volume 22, February 1939 SUMMARY OF AN INVESTIGATION OF THE COMPOSITION OF A MIDCONTINENT PETROLEUM DISTILLATE, BOILING BETWEEN 100° AND 130° C.l By Robert T. Leslie 2 ABSTRACT The results of 7 years of investigation of the composition of the naphtha fraction of petroleum boiling between 1000 and 1300 C are summarized. Of the 130 liters found to boil in this range after the first laboratory distillation, the constitu ents of about one-sixth of the volume were identified or characterized by physical properties, isolated or concentrated, and their concentration in the petroleum roughly estimated. About one-fourth of the volume remains as unidentified distillate. This is composed in part of material of which the constitution is only predicted (about one-twelfth of the volume) and of additional quantities of the constituents which were isolated previously (about one-sixth of the volume). The remainder was lost during the investigation or distilled out of the range on subsequent distillations. Photomicrographic studies were made of those con centrations of distillate which could be crystallized. There is given a bibliography of the physical constant s of hydrocarbons reported to boil within this range. CONTENTS Page I. Introduction __ __ ________ __ ____ ______ ________________________ ____ 153 II. Study of the composition of the distillate ___ ___________ __ ___ ________ 164 1. Distillate between 98 0 and 102 0 C ___ _________ ________________ 164 2. Distillate between 1030 and 106 0 C _ _ _ _ __ __ __ __ __ _ __ _ _ _ __ __ _ __ 164 3. -

Directive Effects in Abstraction Reactions of the Phenyl Radical Robert Frederick Bridger Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1963 Directive effects in abstraction reactions of the phenyl radical Robert Frederick Bridger Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Bridger, Robert Frederick, "Directive effects in abstraction reactions of the phenyl radical " (1963). Retrospective Theses and Dissertations. 2336. https://lib.dr.iastate.edu/rtd/2336 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been 63-5170 microfilmed exactly as received BRIDGER, Robert Frederick, 1934- DIRECTIVE EFFECTS IN ABSTRACTION RE ACTIONS OF THE PHENYL RADICAL. Iowa State University of Science and Technology Ph.D., 1963 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan DIRECTIVE EFFECTS IN ABSTRACTION REACTIONS OF THE PHENYL RADICAL by Robert Frederick Bridger A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Organic Chemistry Approved: Signature was redacted for privacy. In Charge of Major Work Signature was redacted for privacy. Head of Major Depart me6jb Signature was redacted for privacy. Iowa State University Of Science and Technology Ames, Iowa 1963 ii TABLE OF CONTENTS Page INTRODUCTION 1 LITERATURE REVIEW 3 RESULTS 6 DISCUSSION 36 EXPERIMENTAL 100 SUMMARY 149 REFERENCES CITED 151 ACKNOWLEDGEMENTS 158 iii LIST OF FIGURES Page Figure 1. -
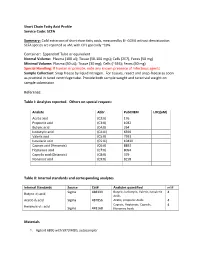
Short Chain Fatty Acid Profile Service Code: SCFA
Short Chain Fatty Acid Profile Service Code: SCFA Summary: Cold extraction of short chain fatty acids, measured by EI- GCMS without derivatization. SCFA species are reported as uM, with CV's generally ~10%. Container: Eppendorf Tube or equivalent Normal Volume: Plasma (100 ul); Tissue (50-100 mgs); Cells (2E7), Feces (50 mg) Minimal Volume: Plasma (50 uL); Tissue (30 mg); Cells (~5E6); Feces (40 mg) Special Handling: If human or primate, note any known presence of infectious agents. Sample Collection: Snap freeze by liquid nitrogen. For tissues, resect and snap-freeze as soon as practical in tared centrifuge tube. Provide both sample weight and tared vial weight on sample submission Reference: Table I: Analytes reported. Others on special request: Analyte Abbr. PubCHEM LOQ(uM) Acetic acid (C2:0) 176 Propionic acid (C3:0) 1032 Butyric acid (C4:0) 264 Isobutyric acid (C4:0i) 6590 Valeric acid (C5:0) 7991 Isovaleric acid (C5:0i) 10430 Caproic acid (Hexanoic) (C6:0) 8892 Heptanoic acid (C7:0) 8094 Caprylic acid (Octanoic) (C8:0) 379 Nonanoic acid (C9:0) 8158 Table II: Internal standards and corresponding analytes Internal Standards Source Cat# Analytes quantified mM Sigma 488399 Butyric, Isobutyric, Valeric, Isovaleric 4 Butyric-d7 acid Acids Acetic-d3 acid Sigma 487856 Acetic, propionic Acids 4 Caproic, Heptanoic, Caprylic, 4 Hexanoic-d11 acid Sigma 448168 Nonanoic Acids Materials 1. Agilent 6890 with 5973 MSD, autosampler 2. Vortexer 3. Refrigerated centrifuge, capable of 13,000g with eppendorf tube compatible rotor 4. ice bucket, ice 5. Balance 6. Prepared stock solutions of short chain fatty acid standards and isotope-labeled short chain fatty acid internal standards. -

Federal Register/Vol. 81, No. 250/Thursday, December 29, 2016
95886 Federal Register / Vol. 81, No. 250 / Thursday, December 29, 2016 / Rules and Regulations C. Regulatory Flexibility Act (RFA) I. National Technology Transfer and ENVIRONMENTAL PROTECTION Advancement Act (NTTAA) AGENCY This action is not subject to the RFA. This rulemaking does not involve The RFA applies only to rules subject to 40 CFR Part 180 notice-and-comment rulemaking technical standards. requirements under the APA, 5 U.S.C. [EPA–HQ–OPP–2016–0007 and EPA–HQ– J. Executive Order 12898: Federal OPP–2016–0008; FRL–9950–40] 553, or any other statute. This rule is not Actions To Address Environmental subject to notice-and-comment Justice in Minority Populations and Isobutyl Acetate and Isobutyric Acid; requirements because the agency has Low-Income Populations Exemption From the Requirement of a invoked the APA ‘‘good cause’’ The EPA believes that this action is Tolerance exemption under 5 U.S.C. 553(b). not subject to Executive Order 12898 (59 AGENCY: Environmental Protection FR 7629, February 16, 1994) because it D. Unfunded Mandates Reform Act Agency (EPA). (UMRA) does not establish an environmental health or safety standard. This good ACTION: Final rule. This action does not contain any cause final action simply extends the SUMMARY: This regulation establishes unfunded mandate of $100 million or date for the EPA to take action on a more as described in UMRA, 2 U.S.C. exemptions from the requirement of a petition and does not have any impact tolerance for residues of isobutyl acetate 1531–1538, and does not significantly or on human health or the environment. -
![[Agr. Biol. Chem., Vol. 36, No. 1, P. 112---119, 1972] Studies of the Peptide Antibiotic Suzukacillin Part II by Takaaki OOKA An](https://docslib.b-cdn.net/cover/9902/agr-biol-chem-vol-36-no-1-p-112-119-1972-studies-of-the-peptide-antibiotic-suzukacillin-part-ii-by-takaaki-ooka-an-399902.webp)
[Agr. Biol. Chem., Vol. 36, No. 1, P. 112---119, 1972] Studies of the Peptide Antibiotic Suzukacillin Part II by Takaaki OOKA An
[Agr. Biol. Chem., Vol. 36, No. 1, p. 112---119,1972] Studies of the Peptide Antibiotic Suzukacillin Part II By Takaaki OOKA and Isao TAKEDA TechnicalResearch Laboratory, Asahi ChemicalIndustry Co., Ltd., Nakadai-cho3-27, Itabashi-kuTokyo ReceivedJuly 26, 1971 Suzukacillin produced by Trichoderma viride 6301 strain was purified and shown to be separated into two components A and B on thin-layer chromatography. The component A was isolated and crystallized from the mixture of components by alumina column chro matography. The component A is composed of six amino acids, Gly, Glu, Ala, Pro, Val, Leu and an unknown amino acid. This unknown amino acid was identified as a-amino isobutyric acid. It is supposed that a-amino isobutyrie acid is biosynthesized mainly from L-valine by the isotopic experiments. Suzukacillin formation by Trichoderma viride 6301 was stimulated by the addition of L-Asn, GABA, L-Ser, Gly and L-Arg into the medium. Trichoderma viride 6301 strain producing Suzu ments were purchased as reagents from Tokyo Kasei kacillin was isolated by the author's laboratory. Co., Ltd. The following radioactive compounds were This organism accumulated noted amount of surplied by Dai-ichi Chemical Co., Ltd., with the in the peptide antibiotic. It was detected on dicated specific activities (ƒÊCi per mmole): Starch- TLC that the peptide antibiotic Suzukacillin (U)14C (30.8), pyruvic acid-(U)-14C (31.5), glycine- consists of two components A and B. A is (U)14C (45.2), L-alanine-(U)-14C (107), L-glutamic acid- (U)-14C (165), L-proline-(U)-14C (165), L-valine-(U)-14C a major and B is a minor component. -

Influence of Sargassum Horneri Mitigating Odorous Gas Emissions from Swine Manure Storage Facilities
sustainability Article Influence of Sargassum horneri Mitigating Odorous Gas Emissions from Swine Manure Storage Facilities Lavanya Madhavaraj 1, Ho-Dong Lim 1, Kong-Min Kim 1, Dae-Hyuk Kim 2 and Gui Hwan Han 1,* 1 Center for Industrialization of Agricultural and Livestock Microorganisms (CIALM), 241, Cheomdangwahag-ro, Jeongeup-si 56212, Korea; [email protected] (L.M.); [email protected] (H.-D.L.); [email protected] (K.-M.K.) 2 Department of Molecular Biology, Institute for Molecular Biology and Genetics, Jeonbuk National University, Jeonju 561–756, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-63-536-6713; Fax: +82-63-536-6003 Received: 4 August 2020; Accepted: 11 September 2020; Published: 15 September 2020 Abstract: Manures from livestock industries and farmyards should be managed for land application. Currently, a deep pit or barn system is adopted by many swine farms for manure management, therefore releasing harmful gases and rising the total global emissions of GHGs. This research focuses on the effectiveness of the brown seaweed Sargassum horneri as a masking agent to mitigate odor-generating gaseous pollutants and reduce the emissions of volatile fatty acids (VFAs) from swine manure storage facilities. Using an optimized procedure, we compared the gaseous emissions from two manure storage barns, one containing swine manure masked with S. horneri and the other without masking as a control, over a 30-day period. The results showed that, compared to the control, seaweed masking significantly reduced the sulfide and VFA contents. Furthermore, reductions of 99.48% in H S, 60 5.21% in NH and 74.28 2.14% in gaseous amine emissions were observed 2 ± 3 ± within the experimental period. -

BGA244 Binary Gas Analyzer User Manual
BGA244 Binary Gas Analyzer User Manual Revision 1.10 Certification Stanford Research Systems certifies that this product met its published specification at the time of shipment. Warranty This Stanford Research Systems product is warranted against defects in materials and workmanship for a period of one (1) year from the date of shipment. Service For warranty service or repair, this product must be returned to a Stanford Research Systems authorized service facility. Contact Stanford Research Systems or an authorized representative for a RMA (Return Material Authorization) Number before returning this product for repair. These are available at www.thinksrs.com under Support, Repair/Calibration. All users returning a BGA244 back to the factory for repair and/or service must submit a correctly completed “Declaration of Contamination of Equipment” form, available as part of the RMA process. The SRS personnel carrying out repair and service of the BGA244 must be informed of the condition of the components prior to any work being performed. Warning All returns to SRS must be free of harmful, corrosive, radioactive or toxic materials. Dedication In memory of Jim Williams, 1948 - 2011: Legendary Author and Analog Design Guru Information in this document is subject to change without notice. Copyright © Stanford Research Systems, Inc., 2016-2018. All rights reserved. Stanford Research Systems, Inc. 1290-C Reamwood Avenue Sunnyvale, California 94089 Phone: (408) 744-9040 Fax: (408) 744-9049 Email: [email protected] www.thinksrs.com Printed in the -

Revised Group Additivity Values for Enthalpies of Formation (At 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds
Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds Cite as: Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 Submitted: 17 January 1996 . Published Online: 15 October 2009 N. Cohen ARTICLES YOU MAY BE INTERESTED IN Additivity Rules for the Estimation of Molecular Properties. Thermodynamic Properties The Journal of Chemical Physics 29, 546 (1958); https://doi.org/10.1063/1.1744539 Critical Evaluation of Thermochemical Properties of C1–C4 Species: Updated Group- Contributions to Estimate Thermochemical Properties Journal of Physical and Chemical Reference Data 44, 013101 (2015); https:// doi.org/10.1063/1.4902535 Estimation of the Thermodynamic Properties of Hydrocarbons at 298.15 K Journal of Physical and Chemical Reference Data 17, 1637 (1988); https:// doi.org/10.1063/1.555814 Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 25, 1411 © 1996 American Institute of Physics for the National Institute of Standards and Technology. Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds N. Cohen Thermochemical Kinetics Research, 6507 SE 31st Avenue, Portland, Oregon 97202-8627 Received January 17, 1996; revised manuscript received September 4, 1996 A program has been undertaken for the evaluation and revision of group additivity values (GAVs) necessary for predicting, by means of Benson's group additivity method, thermochemical properties of organic molecules. This review reports on the portion of that program dealing with GAVs for enthalpies of formation at 298.15 K (hereinafter abbreviated as 298 K) for carbon-hydrogen and carbon-hydrogen-oxygen compounds. -

Extraction of Benzoic Acid Dr
CHEM 2423 Extraction of Benzoic Acid Dr. Pahlavan EXPERIMENT 6 - Extraction Determination of Distribution Coefficient Purpose: a) To purify samples of organic compounds that are solids at room temperature b) To dissociate the impure sample in the minimum amount of an appropriate hot solvent Equipment / Materials: Benzoic acid solution ring& ring stand burette burette clamp spatula 0.1 M NaOH (or 0.02 M) solution separatory funnel methylene dichloride 125 ml Erlemeyer flask 10 and 50 mL graduated cylinders 50 and 100 mL beakers funnel Discussion: Crystallization, purification, and isolation (may only be restricted to a solid) are insufficient ways to separate mixtures of compounds. Extraction is the recovery of a substance from a mixture by bringing it into contact with a solvent, which dissolves the desired material. Partitioning is the separation between two distinct phases (immiscible liquids) and also called fractional separation. Like recrystallization and distillation, extraction is a separation technique frequently employed in the laboratory to isolate one or more components from a mixture. Unlike recrystallization and distillation, it does not yield a pure product; thus, the former techniques may be required to purify a product isolated by extraction. In the technical sense extraction is based on the principle of the equilibrium distribution of a substance (solute) between two immiscible phases, one of which is usually a solvent. The solvent need not be a pure liquid but may be a mixture of several solvents or a solution of some chemical reagent that will react with one or more components of the mixture being extracted to form a new substance soluble in the solution. -

Cigarette Additives, Carcinogens and Chemicals Nicotine
Cigarette Additives, Carcinogens and Chemicals Nicotine A Destructive Natural Pesticide Which ... Is extremely addictive when smoked Is extremely addictive when chewed Causes addiction as permanent as Is harder to quit than heroin or cocaine alcoholism Is not medicine and its use not therapy Is ineffective as a stand-alone quitting aid Prevents pre-cancerous cells from dying Accelerates cancer tumor growth rates Contributes to artery hardening Has a metabolite which may cause cancer May kill brain cells and impair memory Is linked to lung cancer Likely causes brain damage and Is also a fetus destroying teratogen depression Kills half of adult smokers 13-14 years Is beat by never taking another puff or early chew! 81 Cancer Causing Chemicals Have So Far Been Identified in Cigarettes Acetaldehyde Acetamide Acrylamide Acrylonitrile 2-Amino-3,4-dimethyl-3H-imidazo[4,5-f]quinoline (MeIQ) 3-Amino-1,4-dimethyl-5H-pyrido [4,3-b]indole (Trp-P-1) 2-Amino-l-methyl-6-phenyl-1H-imidazo [4,5-b]pyridine (PhlP) 2-Amino-6-methyldipyrido[1,2-a:3',2'-d]imidazole (Glu-P-1) 3-Amino-l-methyl-5H-pyrido {4,3-b]indole (Trp-P-2 2-Amino-3-methyl-9H-pyrido[2,3-b]indole (MeAaC) 2-Amino-9H-pyrido[2,3-b]indole (AaC) 4-Aminobiphenyl 2-Aminodipyrido[1,2-a:3',2'-d]imidazole (Glu-P-2) 0-Anisidine Arsenic Benz[a]anthracene Benzene Benzo[a]pyrene Benzo[b]fluoranthene Benzo[j]fluoranthene Benzo[k]fluoranthene Benzo[b]furan Beryllium 1,3-Butadiene Cadmium Catechol (1,2-benzenediol) p-Chloroaniline Chloroform Cobalt p,p'-DDT Dibenz[a,h]acridine Dibenz[a,j]acridine Dibenz(a,h)anthracene -

WIDESPREAD OCCURRENCE of SPECIFIC, HIGH AFFINITY BINDING SITES for AMINO ACIDS Stephen C
Vol. 80, No. 1, 1978 BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS January 13, 1978 Pages 161-168 WIDESPREAD OCCURRENCE OF SPECIFIC, HIGH AFFINITY BINDING SITES FOR AMINO ACIDS Stephen C. Bondy and Marilyn E. Harrington Departments of Neurology and Pharmacology University of Colorado Medical Center, 42DO East Ninth Avenue Denver, Colorado 80262 Received September 27, 1977 The high affinity binding of several amino acids to various membrane and protein preparations has been measured. Binding of radioactive amino acids suspected of being neurotransmitters and also of leucine and tyrosine to brain, liver and heart muscle membranes was saturable, reversible and stereospecific. Similar characteristics were found using chloroform-methanol extracted brain tissue and heat denatured albumin. Compounds thought to act as blockers of postsynaptic binding such as strychnine, bicuculline and kainic acid did not inhibit binding. Thus, specific high affinity interactions between amino acids and proteins are widespread and largely unrelated to neurotransmission. INTRODUCTION The affinity binding of several amino acids such as glutamic acid, glycine and gamma-aminobutyric acid has been reported in membrane preparations of brain (l-4). This binding has been used as a measure of postsynaptic sites specific for a particular amino acid neurotransmitter. Criteria used to establish the identity of these sites include: l) specificity of the interaction and also stereospecific selectivity in several cases; 2) saturable, reversible binding kinetics with a high affinity between membrane and ligand; 3) independence of binding on the presence of the sodium ion (5); 4) no energy dependence of the interaction, temperature should not have a major effect on binding velocity; 5) physiological relevance and appropriate inhibition by known antagonists. -
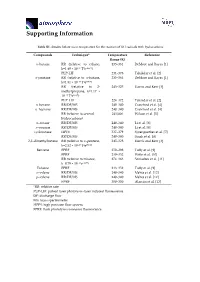
Supporting Information
Supporting Information Table S1. Studies below room temperature for the reaction of OH radicals with hydrocarbons. Compounds Technique* Temperature Reference Range (K) n-butane RR (relative to ethane, 235–361 DeMore and Bayes [1] k=1.49 × 10−17 T2e−499/T) PLP-LIF 231–378 Talukdar et al. [2] n-pentane RR (relative to n-butane, 233–364 DeMore and Bayes [1] k=1.81 × 10−17 T2e114/T) RR (relative to 2- 243–325 Harris and Kerr [3] methylpropane, k=1.17 × 10−17 T2e213/T) PLP–LIF 224–372 Talukdar et al. [2] n–hexane RR/DF/MS 240–340 Crawford et al. [4] n–heptane RR/DF/MS 240–340 Crawford et al. [4] RR (relative to several 241/406 Wilson et al. [5] hydrocarbons) n–octane RR/DF/MS 240–340 Li et al. [6] n–nonane RR/DF/MS 240–340 Li et al. [6] cyclooctane HPFS 237–379 Sprengnether et al. [7] RR/DF/MS 240–340 Singh et al. [8] 2,2–dimethylbutane RR (relative to n–pentane, 245–328 Harris and Kerr [3] k=2.52 × 10–17 T2e158/T) Benzene FPRF 250–298 Tully et al. [9] FPRF 239–352 Witte et al. [10] RR (relative to toluene, 274–363 Semadeni et al. [11] k=0.79 × 10–12 e614/T) Toluene FPRF 213–352 Tully et al. [9] o–xylene RR/DF/MS 240–340 Mehta et al. [12] p–xylene RR/DF/MS 240–340 Mehta et al. [12] FPRF 300–350 Alarcón et al. [13] *RR: relative rate PLP–LIF: pulsed laser photolysis–laser induced fluorescence DF: discharge flow MS: mass spectrometry HPFS: high pressure flow system FPRF: flash photolysis resonance fluorescence Heating control Temperature Regulator Holes to release extra N 2 Glass lined tube Temperature sensor From smog Pump chamber Magnetic Magnetic Valve 1 Valve 2 GC column Magnetic Cooling jacket Carrier gas FID Valve (He) Dewar tank of liquid N 2 Figure S1.