SUMMARY of an INVESTIGATION of the COMPOSITION of a MIDCONTINENT PETROLEUM DISTILLATE, BOILING BETWEEN 100° and 130° C.L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Directive Effects in Abstraction Reactions of the Phenyl Radical Robert Frederick Bridger Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1963 Directive effects in abstraction reactions of the phenyl radical Robert Frederick Bridger Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Bridger, Robert Frederick, "Directive effects in abstraction reactions of the phenyl radical " (1963). Retrospective Theses and Dissertations. 2336. https://lib.dr.iastate.edu/rtd/2336 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been 63-5170 microfilmed exactly as received BRIDGER, Robert Frederick, 1934- DIRECTIVE EFFECTS IN ABSTRACTION RE ACTIONS OF THE PHENYL RADICAL. Iowa State University of Science and Technology Ph.D., 1963 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan DIRECTIVE EFFECTS IN ABSTRACTION REACTIONS OF THE PHENYL RADICAL by Robert Frederick Bridger A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Organic Chemistry Approved: Signature was redacted for privacy. In Charge of Major Work Signature was redacted for privacy. Head of Major Depart me6jb Signature was redacted for privacy. Iowa State University Of Science and Technology Ames, Iowa 1963 ii TABLE OF CONTENTS Page INTRODUCTION 1 LITERATURE REVIEW 3 RESULTS 6 DISCUSSION 36 EXPERIMENTAL 100 SUMMARY 149 REFERENCES CITED 151 ACKNOWLEDGEMENTS 158 iii LIST OF FIGURES Page Figure 1. -

BGA244 Binary Gas Analyzer User Manual
BGA244 Binary Gas Analyzer User Manual Revision 1.10 Certification Stanford Research Systems certifies that this product met its published specification at the time of shipment. Warranty This Stanford Research Systems product is warranted against defects in materials and workmanship for a period of one (1) year from the date of shipment. Service For warranty service or repair, this product must be returned to a Stanford Research Systems authorized service facility. Contact Stanford Research Systems or an authorized representative for a RMA (Return Material Authorization) Number before returning this product for repair. These are available at www.thinksrs.com under Support, Repair/Calibration. All users returning a BGA244 back to the factory for repair and/or service must submit a correctly completed “Declaration of Contamination of Equipment” form, available as part of the RMA process. The SRS personnel carrying out repair and service of the BGA244 must be informed of the condition of the components prior to any work being performed. Warning All returns to SRS must be free of harmful, corrosive, radioactive or toxic materials. Dedication In memory of Jim Williams, 1948 - 2011: Legendary Author and Analog Design Guru Information in this document is subject to change without notice. Copyright © Stanford Research Systems, Inc., 2016-2018. All rights reserved. Stanford Research Systems, Inc. 1290-C Reamwood Avenue Sunnyvale, California 94089 Phone: (408) 744-9040 Fax: (408) 744-9049 Email: [email protected] www.thinksrs.com Printed in the -

Revised Group Additivity Values for Enthalpies of Formation (At 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds
Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds Cite as: Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 Submitted: 17 January 1996 . Published Online: 15 October 2009 N. Cohen ARTICLES YOU MAY BE INTERESTED IN Additivity Rules for the Estimation of Molecular Properties. Thermodynamic Properties The Journal of Chemical Physics 29, 546 (1958); https://doi.org/10.1063/1.1744539 Critical Evaluation of Thermochemical Properties of C1–C4 Species: Updated Group- Contributions to Estimate Thermochemical Properties Journal of Physical and Chemical Reference Data 44, 013101 (2015); https:// doi.org/10.1063/1.4902535 Estimation of the Thermodynamic Properties of Hydrocarbons at 298.15 K Journal of Physical and Chemical Reference Data 17, 1637 (1988); https:// doi.org/10.1063/1.555814 Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 25, 1411 © 1996 American Institute of Physics for the National Institute of Standards and Technology. Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds N. Cohen Thermochemical Kinetics Research, 6507 SE 31st Avenue, Portland, Oregon 97202-8627 Received January 17, 1996; revised manuscript received September 4, 1996 A program has been undertaken for the evaluation and revision of group additivity values (GAVs) necessary for predicting, by means of Benson's group additivity method, thermochemical properties of organic molecules. This review reports on the portion of that program dealing with GAVs for enthalpies of formation at 298.15 K (hereinafter abbreviated as 298 K) for carbon-hydrogen and carbon-hydrogen-oxygen compounds. -
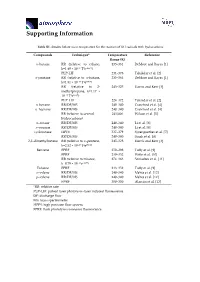
Supporting Information
Supporting Information Table S1. Studies below room temperature for the reaction of OH radicals with hydrocarbons. Compounds Technique* Temperature Reference Range (K) n-butane RR (relative to ethane, 235–361 DeMore and Bayes [1] k=1.49 × 10−17 T2e−499/T) PLP-LIF 231–378 Talukdar et al. [2] n-pentane RR (relative to n-butane, 233–364 DeMore and Bayes [1] k=1.81 × 10−17 T2e114/T) RR (relative to 2- 243–325 Harris and Kerr [3] methylpropane, k=1.17 × 10−17 T2e213/T) PLP–LIF 224–372 Talukdar et al. [2] n–hexane RR/DF/MS 240–340 Crawford et al. [4] n–heptane RR/DF/MS 240–340 Crawford et al. [4] RR (relative to several 241/406 Wilson et al. [5] hydrocarbons) n–octane RR/DF/MS 240–340 Li et al. [6] n–nonane RR/DF/MS 240–340 Li et al. [6] cyclooctane HPFS 237–379 Sprengnether et al. [7] RR/DF/MS 240–340 Singh et al. [8] 2,2–dimethylbutane RR (relative to n–pentane, 245–328 Harris and Kerr [3] k=2.52 × 10–17 T2e158/T) Benzene FPRF 250–298 Tully et al. [9] FPRF 239–352 Witte et al. [10] RR (relative to toluene, 274–363 Semadeni et al. [11] k=0.79 × 10–12 e614/T) Toluene FPRF 213–352 Tully et al. [9] o–xylene RR/DF/MS 240–340 Mehta et al. [12] p–xylene RR/DF/MS 240–340 Mehta et al. [12] FPRF 300–350 Alarcón et al. [13] *RR: relative rate PLP–LIF: pulsed laser photolysis–laser induced fluorescence DF: discharge flow MS: mass spectrometry HPFS: high pressure flow system FPRF: flash photolysis resonance fluorescence Heating control Temperature Regulator Holes to release extra N 2 Glass lined tube Temperature sensor From smog Pump chamber Magnetic Magnetic Valve 1 Valve 2 GC column Magnetic Cooling jacket Carrier gas FID Valve (He) Dewar tank of liquid N 2 Figure S1. -

Chapter 21 Organic Chemistry
CHAPTER 21 ORGANIC CHEMISTRY Hydrocarbons 1. A hydrocarbon is a compound composed of only carbon and hydrogen. A saturated hydro- carbon has only carbon-carbon single bonds in the molecule. An unsaturated hydrocarbon has one or more carbon-carbon multiple bonds but may also contain carbon-carbon single bonds. A normal hydrocarbon has one chain of consecutively bonded carbon atoms. A branched hydrocarbon has at least one carbon atom not bonded to the end carbon of a chain of consecutively bonded carbon atoms. Instead, at least one carbon atom forms a bond to an inner carbon atom in the chain of consecutively bonded carbon atoms. 2. To determine the number of hydrogens bonded to the carbons in cyclic alkanes (or any alkane where they may have been omitted), just remember that each carbon has four bonds. In cycloalkanes, only the C−C bonds are shown. It is assumed you know that the remaining bonds on each carbon are C−H bonds. The number of C−H bonds is that number required to give the carbon four total bonds. 3. In order to form, cyclopropane and cyclobutane are forced to form bond angles much smaller than the preferred 109.5° bond angles. Cyclopropane and cyclobutane easily react in order to obtain the preferred 109.5° bond angles. 4. Aromatic hydrocarbons are a special class of unsaturated hydrocarbons based on the benzene ring. Benzene has the formula C6H6. It is a planar molecule (all atoms are in the same plane). Each carbon in benzene is attached to three other atoms; it exhibits trigonal planar geometry with 120° bond angles. -

Aircraft Fuels and Propellants
The AAF Scientific Advisory Group was activated late in 1944 by General of the Army H. H. Arnold. He se cured the services of Dr. Theodore von Karman, re nowned scientist and consultant in aeronautics, who agreed to organize and direct the group. Dr. von Karman gathered about him a group of Ameri can scientists from every field of research having a bearing on air power. These men then analyzed im portant developments in the basic sciences, both here and abroad, and attempted to evaluate the effects of their application to air power. This volume is one of a group of reports made to the Army Air Forces by the Scientific Advisory Group. Thil document contolnl Information affecting the notional defenle of the United Statel within the meaning of the Espionage Ad, SO U. S. C., 31 and 32, 01 amended. Its tronsmiulon or the revelation of Its contents In any manner to on unauthorized person II prohibited by low. AAF SCIE.TIFIC ADVISORY GROUP Dr. Th. von Karman Director Colonel F. E. Glantz berg Dr. H. L. Dryden Deputy Director, Military Deputy Director, Scientific Lt Col G. T. McHugh, Executive Capt C. H. Jackson, Jr., Secretary CONSULTANTS Dr. C. W. Bray Dr. A. J. Stosick Dr. L. A. DuBridge Dr. W. J. Sweeney Dr. Pol Duwez Dr. H. S. Tsien Dr. G. Gamow Dr. G. E. Valley Dr. I. A. Getting Dr. F. L. Wattendorf Dr. L. P. Hammett Dr. F. Zwicky Dr. W. S. Hunter Dr. V. K. Zworykin Dr. I. P. Krick Colonel D. N. Yates Dr. -

"Hydrocarbons," In: Ullmann's Encyclopedia of Industrial Chemistry
Article No : a13_227 Hydrocarbons KARL GRIESBAUM, Universit€at Karlsruhe (TH), Karlsruhe, Federal Republic of Germany ARNO BEHR, Henkel KGaA, Dusseldorf,€ Federal Republic of Germany DIETER BIEDENKAPP, BASF Aktiengesellschaft, Ludwigshafen, Federal Republic of Germany HEINZ-WERNER VOGES, Huls€ Aktiengesellschaft, Marl, Federal Republic of Germany DOROTHEA GARBE, Haarmann & Reimer GmbH, Holzminden, Federal Republic of Germany CHRISTIAN PAETZ, Bayer AG, Leverkusen, Federal Republic of Germany GERD COLLIN, Ruttgerswerke€ AG, Duisburg, Federal Republic of Germany DIETER MAYER, Hoechst Aktiengesellschaft, Frankfurt, Federal Republic of Germany HARTMUT Ho€KE, Ruttgerswerke€ AG, Castrop-Rauxel, Federal Republic of Germany 1. Saturated Hydrocarbons ............ 134 3.7. Cumene ......................... 163 1.1. Physical Properties ................ 134 3.8. Diisopropylbenzenes ............... 164 1.2. Chemical Properties ............... 134 3.9. Cymenes; C4- and C5-Alkylaromatic 1.3. Production ....................... 134 Compounds ...................... 165 1.3.1. From Natural Gas and Petroleum . .... 135 3.10. Monoalkylbenzenes with Alkyl Groups 1.3.2. From Coal and Coal-Derived Products . 138 >C10 ........................... 166 1.3.3. By Synthesis and by Conversion of other 3.11. Diphenylmethane .................. 167 Hydrocarbons . .................. 139 4. Biphenyls and Polyphenyls .......... 168 1.4. Uses ............................ 140 4.1. Biphenyl......................... 168 1.5. Individual Saturated Hydrocarbons ... 142 4.2. Terphenyls...................... -

Flammability Limits, Flash Points, and Their Consanguinity: Critical Analysis, Experimental Exploration, and Prediction
Brigham Young University BYU ScholarsArchive Theses and Dissertations 2010-06-25 Flammability Limits, Flash Points, and Their Consanguinity: Critical Analysis, Experimental Exploration, and Prediction Jeffrey R. Rowley Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Chemical Engineering Commons BYU ScholarsArchive Citation Rowley, Jeffrey R., "Flammability Limits, Flash Points, and Their Consanguinity: Critical Analysis, Experimental Exploration, and Prediction" (2010). Theses and Dissertations. 2233. https://scholarsarchive.byu.edu/etd/2233 This Dissertation is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Flammability Limits, Flash Points, and their Consanguinity: Critical Analysis, Experimental Exploration, and Prediction Jef Rowley A dissertation submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Doctor of Philosophy W. Vincent Wilding, Chair Richard L. Rowley Larry L. Baxter Thomas A. Knotts David O. Lignell Department of Chemical Engineering Brigham Young University August 2010 Copyright © 2010 Jef Rowley All Rights Reserved ABSTRACT Flammability Limits, Flash Points, and their Consanguinity: Critical Analysis, Experimental Exploration, and Prediction Jef Rowley Department of Chemical Engineering Doctor of Philosophy Accurate flash point and flammability limit data are needed to design safe chemical processes. Unfortunately, improper data storage and reporting policies that disregard the temperature dependence of the flammability limit and the fundamental relationship between the flash point and the lower flammability limit have resulted in compilations filled with erroneous values. -

Calculation of the Isobaric Heat Capacities of the Liquid and Solid Phase of Organic Compounds at and Around 298.15 K Based on Their “True” Molecular Volume
molecules Article Calculation of the Isobaric Heat Capacities of the Liquid and Solid Phase of Organic Compounds at and around 298.15 K Based on Their “True” Molecular Volume Rudolf Naef Department of Chemistry, University of Basel, 4003 Basel, Switzerland; [email protected]; Tel.: +41-61-9119273 Received: 28 March 2019; Accepted: 20 April 2019; Published: 24 April 2019 Abstract: A universally applicable method for the prediction of the isobaric heat capacities of the liquid and solid phase of molecules at 298.15 K is presented, derived from their “true” volume. The molecules’ “true” volume in A3 is calculated on the basis of their geometry-optimized structure and the Van-der-Waals radii of their constituting atoms by means of a fast numerical algorithm. Good linear correlations of the “true” volume of a large number of compounds encompassing all classes and sizes with their experimental liquid and solid heat capacities over a large range have been found, although noticeably distorted by intermolecular hydrogen-bond effects. To account for these effects, the total amount of 1303 compounds with known experimental liquid heat capacities has been subdivided into three subsets consisting of 1102 hydroxy-group-free compounds, 164 monoalcohols/monoacids, and 36 polyalcohols/polyacids. The standard deviations for Cp(liq,298) were 20.7 J/mol/K for the OH-free compunds, 22.91 J/mol/K for the monoalcohols/monoacids and 16.03 J/mol/K for the polyols/polyacids. Analogously, 797 compounds with known solid heat capacities have been separated into a subset of 555 OH-free compounds, 123 monoalcohols/monoacids and 119 polyols/polyacids. -

Compound Other Saturated Aliphatic Hydrocarbons C6-C8 Data Collection Sheet (1/4)
Compound Other saturated aliphatic hydrocarbons C6-C8 Data collection sheet (1/4) See Annex 1 for existing human health-based CLP See Table 1 for CAS numbers in the factsheet classifications in the factsheet Organisation name AgBB ANSES BAuA ECHA ECHA DNEL(general population, long-term, inhalation, systemic) DNEL(general population, long-term, [Hydrocarbons, C6-C7, n- NIK (=LCI) CLI (=LCI) OEL (aliphatic C6-C8) inhalation, systemic) alkanes, isoalkanes, (octane & isooctane) cyclics, <5% n-hexane” Risk value name (EC number: 921-024-6)] Risk value (µg/m3) 15000 10000 700000 608000 608000 Risk value (ppb) N/A N/A N/A N/A ~130000 Reference period Chronic Chronic Chronic Chronic Chronic Year 2012 1993 2017 2011, updated 2017 2011, updated in 2017 GLP-guideline study GLP-guideline study (OECD guideline 413) as (OECD guideline 413) as Key study N/A N/A N/A reported in the reported in the registration dossiers for registration dossier octane and isooctane Subchronic inhalation Subchronic inhalation Study type N/A N/A N/A study study Species N/A N/A N/A Rat Rat 6 h/d, 5 d/wk for 13 6 h/d, 5 d/wk for 13 Duration of exposure N/A N/A N/A weeks weeks Value based on provisional The group limit value Subchronic toxicity or Subchronic toxicity or OEL by the German BAuA is based on the 700 neurotoxic effects of neurotoxic effects of Critical effect for C5-C8 aliphatic N/A mg/m³ (200 ppm) “light alkylate naphtha “light alkylate naphtha hydrocarbons in 2012 limit value for distillate” (CAS 64741-66- distillate” (CAS 64741-66- (BAuA, 2012). -

Slr2 Displacements and Reductive Coupling of Ketones with Olefins in N,N-Diethylacetamide and N-Ethylpyrrolidone
Illeprinted lrom the.krurnalof Organic('hemistrl', ,14, 2;169 (1979).1 (-'opyrightO 19?9ltv the American (lhemical Society and reprinted bv permissiot-rol'the copyright owner. Slr2 Displacements and Reductive Coupling of Ketones with Olefins in N,N-Diethylacetamide and N-Ethylpyrrolidone Allan F. Sowinskiand GeorgeM. Whitesides* Departmentof Chemistrt',Massachusetts Institute of Technologt',Cambridge, Massachusetts 02139 ReceiuedMarch 8. 1979 N,l/-Diethylacetamide (DEA) and N-ethylpyrrolidone (NEP) are complementaryto hexamethylphosphoric triamide (HMPA), dimethylformamide,and dimethyl sulfoxideas solventsin which to carry out severalreactions commonly conductedin polar, aprotic media. Reactionof neopentyltosylates with lithium halidesin DEA and NEP givesgood yields of neopentylhalides. Ketonesand terminal olefinsare reductivelycoupled to tertiary, alcoholsin fair to good yields in mixtures containing NEP, sodium, and f erf -butyl alcohol;1-hepten-6-one and 1-octen-7-oneare cleanly cyclizedto five- and six-memberedrings, respectively,in good yield by this samemixture. Terminal olefins are reduced to alkanes in fair yield by DEA sodium ferf-butyl alcohol mixtures; di- and tetrasubstituted olefins are resistant to reduction bv this mixture. Hexamethylphosphorictriamide (HMPA) is a dipolar more limited value than HMPA for dissolving-metalre- aprotic solventr'2that is particularl5rvaluable for such duction of olefins. In addition, we describea useful new reactionsas SN2displacement with anionic nucleophilesrr'a reaction which occurs in NEP (but not in HMPA) bv and dissolving-metalreductions.s The routine use of which ketonesand terminal olefins are reductively coupled HMPA has beendiscouraged by the announcementthat to tertiary alcoholsby sodium-ferf-butyl alcohol mixtures. it may be carcinogenic.6In the courseof other studies, we required quantities of several neopentyl halides. Results and Discussion Compoundsof this type had proved in previouswork to Bimolecular Substitution in DEA and NEP. -

4Nomenclature and Conformations of Alkanes
P1: PBU/OVY P2: PBU/OVY QC: PBU/OVY T1: PBU Printer: Bind Rite JWCL234-04 JWCL234-Solomons-v1 December 8, 2009 21:31 4 NOMENCLATURE AND CONFORMATIONS OF ALKANES AND CYCLOALKANES SOLUTIONS TO PROBLEMS 4.1 or or CH3(CH2)5CH3 (CH3)2CHCH2CH2CH2CH3 Heptane 2-Methylhexane or or CH3CH2CH(CH3)CH2CH2CH3 (CH3)3CCH2CH2CH3 3-Methylhexane 2,2-Dimethylpentane or or (CH3CH2)2C(CH3)2 (CH3)2CHCH(CH3)CH2CH3 3,3-Dimethylpentane 2,3-Dimethylpentane or or (CH ) CHCH CH(CH ) 3 2 2 3 2 (CH3CH2)3CH 2,4-Dimethylpentane 3-Ethylpentane or (CH3)3CCH(CH3)2 2,2,3-Trimethylbutane 47 CONFIRMING PAGES P1: PBU/OVY P2: PBU/OVY QC: PBU/OVY T1: PBU Printer: Bind Rite JWCL234-04 JWCL234-Solomons-v1 December 8, 2009 21:31 48 NOMENCLATURE AND CONFORMATIONS OF ALKANES AND CYCLOALKANES 4.2 (d); it represents 3-methylpentane 4.3 CH3CH2CH2CH2CH2CH3 hexane 4.4 (a,b) 2-Methylheptane 3-Methylheptane 4-Methylheptane 2,2-Dimethylhexane 2,3-Dimethylhexane 2,4-Dimethylhexane 2,5-Dimethylhexane 3,3-Dimethylhexane 3,4-Dimethylhexane 3-Ethylhexane 2,2,3-Trimethylpentane 2,2,4-Trimethylpentane 2,3,3-Trimethylpentane 2,3,4-Trimethylpentane 3-Ethyl-2-methylpentane 3-Ethyl-3-methylpentane 2,2,3,3-Tetramethylbutane CONFIRMING PAGES P1: PBU/OVY P2: PBU/OVY QC: PBU/OVY T1: PBU Printer: Bind Rite JWCL234-04 JWCL234-Solomons-v1 December 8, 2009 21:31 NOMENCLATURE AND CONFORMATIONS OF ALKANES AND CYCLOALKANES 49 4.5 (a) Pentyl 1-Methylbutyl 1-Ethylpropyl 2-Methylbutyl 3-Methylbutyl 1,2-Dimethylpropyl 1,1-Dimethylpropyl (b) See the answer to Review Problem 4.1 for the formulas and names of C7H16 isomers.