Monitoring Biologic Therapy in Rheumatoid Arthritis and Psoriatic Arthritis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Sodium Aurothiomalate Injection, Mfr. Std.) 10, 25 and 50 Mg/Ml Anti
PRESCRIBING INFORMATION PrMYOCHRYSINE® (sodium aurothiomalate injection, Mfr. Std.) 10, 25 and 50 mg/mL Anti-Rheumatic Agent (disease modifying) sanofi-aventis Canada Inc. Date of Revision: 2150 St. Elzear Blvd. West November 29, 2007 Laval, Quebec H7L 4A8 Submission Control No.: 114637 s-a Version 2.0 dated NAME OF DRUG PrMYOCHRYSINE® Sodium aurothiomalate injection, Mfr. Std. 10, 25 and 50 mg/mL THERAPEUTIC CLASSIFICATION Anti-rheumatic agent (disease modifying) ACTIONS AND CLINICAL PHARMACOLOGY Sodium aurothiomalate exhibits anti-inflammatory, antiarthritic and immunomodulating effects. The predominant clinical effect of MYOCHRYSINE (sodium aurothiomalate) appears to be suppression of the synovitis in the active stage of the rheumatoid disease. The precise mechanism of action is unknown but it has been suggested that the drug may act by inhibiting cell-mediated and humoral immune mechanisms. Additional modes of action include alteration or inhibition of various enzyme systems, suppression of phagocytic activity of macrophage and polymorphonuclear leukocytes, and alteration of collagen biosynthesis. The metabolic fate of sodium aurothiomalate in humans is unknown but it is believed not to be broken down to elemental gold. It is very highly bound to plasma proteins. Sixty to 90% is excreted very slowly by the renal route while 10 to 40% is eliminated in the feces mostly via biliary secretion. The biologic half-life of gold following a single 50 mg dose of parenteral gold has been reported to range from 6 to 25 days. It increases following successive weekly doses. The appearance of clinical effect is slow. It may take at least 8 weeks to become significant and the maximum benefits may not be achieved for at least 6 months. -

Treatment of Psoriatic Arthritis and Rheumatoid Arthritis with Disease Modifying Drugs — Comparison of Drugs and Adverse Reactions
Treatment of Psoriatic Arthritis and Rheumatoid Arthritis with Disease Modifying Drugs — Comparison of Drugs and Adverse Reactions PHILIP S. HELLIWELL and WILLIAM J. TAYLOR for the CASPAR Study Group ABSTRACT. Objective. Rheumatoid arthritis (RA) and psoriatic arthritis (PsA) are chronic inflammatory diseases of the musculoskeletal system. Although it seems likely that these conditions have a different pathogene- sis, the drugs used to treat them are the same. Our study used a cross-sectional clinical database to com- pare drug use and side-effect profile in these 2 diseases. Methods. The CASPAR study collected data on 588 patients with PsA and 536 controls, 70% of whom had RA. Data on disease modifying drug treatments used over the whole illness were recorded, togeth- er with their outcomes, including adverse events, for RA and PsA. Results. For both diseases methotrexate (MTX) was the most frequently used disease modifying drug (39% of patients with PsA, 30% with RA), with over 70% of patients in both diseases still taking the drug. Other drugs were used with the following frequencies in PsA and RA, respectively: sulfasalazine 22%/13%, gold salts 7%/11%, antimalarial drugs 5%/14%, corticosteroids 10%/17%, and anti-tumor necrosis factor (TNF) drugs 6%/5%. Compared to RA, cyclosporine and anti-TNF agents were less like- ly to be ineffective in PsA. Compared to RA, subjects with PsA were less likely to be taking MTX and more likely to be taking anti-TNF agents. Hepatotoxicity with MTX was more common in PsA, and pul- monary toxicity with MTX was found more often in RA. -

Hedonic Analysis of Arthritis Drugs
This PDF is a selection from an out-of-print volume from the National Bureau of Economic Research Volume Title: Medical Care Output and Productivity Volume Author/Editor: David M. Cutler and Ernst R. Berndt, editors Volume Publisher: University of Chicago Press Volume ISBN: 0-226-13226-9 Volume URL: http://www.nber.org/books/cutl01-1 Publication Date: January 2001 Chapter Title: Hedonic Analysis of Arthritis Drugs Chapter Author: Iain M. Cockburn, Aslam H. Anis Chapter URL: http://www.nber.org/chapters/c7637 Chapter pages in book: (p. 439 - 462) Arthritis Drugs Iain M. Cockburn and Aslam H. Anis 11.1 Introduction This study examines the market for a group of drugs used to treat rheu- matoid arthritis (RA) during the period 1980-92. Rheumatoid arthritis is a painful, debilitating, and progressive disease which affects millions of people worldwide, with very substantial effects on health and the economy. Regrettably, in contrast to some other major health problems such as heart disease, depression, ulcers, and bacterial infections, this is an area where therapeutic innovations have thus far had comparatively little impact on physicians’ ability to reverse the disease. RA currently has no “cure” and the effectiveness of available treatments is limited. Compared to other drug classes the rate of new product introductions has been slow, and, at the time of writing, there have been no breakthroughs of the same order of significance as the discovery and development of SSRIs for treatment of depression, H, antagonists for ulcers, or ACE inhibitors for hypertension. Nonetheless, the market for RA drugs is far from static. -

Elimination Pathways of Fusion Protein and Peptide Drugs
Review Volume 11, Issue 2, 2021, 9139 - 9147 https://doi.org/10.33263/BRIAC112.91399147 Elimination Pathways of Fusion Protein and Peptide Drugs Ali Khodadoust 1 , Hossein Aghamollaei 2 , Ali Mohammad Latifi 1 , Kazem Hasanpour 3 , Mahdi Kamali 4 , Hamid Tebyanian 5 , Gholamreza Farnoosh 1,* 1 Applied Biotechnology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran 2 Chemical Injuries Research Center, Systems biology and Poisonings Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran 3 Sabzevar University of Medical Sciences, School of Medicine, Sabzevar, Iran 4 Nanobiotechnology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran 5 Research Center for Prevention of Oral and Dental Diseases, Baqiyatallah University of Medical Sciences, Tehran, Iran * Correspondence: [email protected]; Scopus Author ID 55855454400 Received: 28.07.2020; Revised: 25.08.2020; Accepted: 27.08.2020; Published: 1.09.2020 Abstract: Fusion proteins have been known as an interesting subject for scientific researches in improving properties or making a new function by synergistically incorporating two protein domains into one complex. Fusion proteins are created by ligation of two or more protein domains in a single polypeptide with functional properties. Improvement of therapeutic function is one of the main goals for developing these products. Elimination from the body is one of the most important points that should be considered in the design and production of fusion proteins. This review describes some of the most important excretion/elimination pathways of fusion peptide and protein drugs as well as serious elimination challenges in the development and manufacturing of fusion proteins. Keywords: Fusion proteins; Therapy; Pharmacokinetics; Serum half-life; Peptide. -

CUE-101, a Novel Fc Fusion Protein for Selective Targeting and Expansion of Anti-Tumor T Cells for Treatment of HPV-Driven Malignancies
CUE-101, a novel Fc fusion protein for selective targeting and expansion of anti-tumor T cells for treatment of HPV-driven malignancies Natasha Girgis1, Steven N. Quayle1, Alyssa Nelson1, Dharma Raj Thapa1, Sandrine Hulot1, Lauren Kraemer1, Miguel Moreta1, Zohra Merazga1, Robert Ruidera1, Dominic Beal1, Mark Haydock1, Jonathan Soriano1, Luke Witt1, Jessica Ryabin1, Emily Spaulding1, John F. Ross1, Saso Cemerski1, Anish Suri1, Rodolfo Chaparro1, Ronald Seidel1, Kenneth J. Pienta2, Mary C. Simcox1 1Cue Biopharma, Cambridge, Massachusetts; 2The James Buchanan Brady Urological Institute and the Department of Urology, Johns Hopkins School of Medicine, Baltimore, Maryland + + Background CUE-101 selectively expands E711-20-specific CD8 T mCUE-101 expands functional antigen-specific CD8 T • Human papilloma virus (HPV) is responsible for 72% of oropharyngeal, 90% of cervical, 90% cells from healthy human PBMCs cells in the tumor and the periphery of anal, and 71% of vulvar, vaginal, or penile cancers, causing significant morbidity and A. B. A. B. C. 9 mortality worldwide. Innovative therapies are urgently needed for these malignancies, Vehicle 100 nM CUE-101 107 15 Peripheral Blood particularly in the largely incurable metastatic setting. 106 10 5 • The E7 oncoprotein is constitutively expressed in HPV-associated cancers, is necessary for 105 ⍺PD-1 5 PE 4 Vehicle mCUE-101 Combo - 10 initiation and maintenance of malignant transformation, and is genetically conserved in 1 cancer (Mirabello 2017). 103 1 1 102 E7-Specific of CD8+ T cells % IFNg+,TNFa+ -

Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation
Review Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation Leonor S. Castro 1,†, Guilherme S. Lobo 1,†, Patrícia Pereira 2 , Mara G. Freire 1 ,Márcia C. Neves 1,* and Augusto Q. Pedro 1,* 1 CICECO–Aveiro Institute of Materials, Chemistry Department, University of Aveiro, Campus Universitário de Santiago, 3810-193 Aveiro, Portugal; [email protected] (L.S.C.); [email protected] (G.S.L.); [email protected] (M.G.F.) 2 Centre for Mechanical Engineering, Materials and Processes, Department of Chemical Engineering, University of Coimbra, Rua Sílvio Lima-Polo II, 3030-790 Coimbra, Portugal; [email protected] * Correspondence: [email protected] (M.C.N.); [email protected] (A.Q.P.) † These authors contributed equally to this work. Abstract: The advent of biopharmaceuticals in modern medicine brought enormous benefits to the treatment of numerous human diseases and improved the well-being of many people worldwide. First introduced in the market in the early 1980s, the number of approved biopharmaceutical products has been steadily increasing, with therapeutic proteins, antibodies, and their derivatives accounting for most of the generated revenues. The success of pharmaceutical biotechnology is closely linked with remarkable developments in DNA recombinant technology, which has enabled the production of proteins with high specificity. Among promising biopharmaceuticals are interferons, first described by Isaacs and Lindenmann in 1957 and approved for clinical use in humans nearly thirty years later. Interferons are secreted autocrine and paracrine proteins, which by regulating several biochemical Citation: Castro, L.S.; Lobo, G.S.; pathways have a spectrum of clinical effectiveness against viral infections, malignant diseases, Pereira, P.; Freire, M.G.; Neves, M.C.; and multiple sclerosis. -
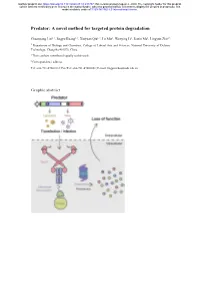
A Novel Method for Targeted Protein Degradation
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.31.231787; this version posted August 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Predator: A novel method for targeted protein degradation Chuanyang Liu1, †, Jingyu Kuang1, †, Xinyuan Qiu1, †, Lu Min1, Wenying Li1, Jiaxin Ma1, Lingyun Zhu1,* 1 Department of Biology and Chemistry, College of Liberal Arts and Sciences, National University of Defense Technology, Changsha 410073, China † These authors contributed equally to this work. ∗ Correspondence address. Tel: +86-731-87001812; Fax/Tel: +86-731-87001801; E-mail: [email protected] Graphic abstract bioRxiv preprint doi: https://doi.org/10.1101/2020.07.31.231787; this version posted August 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract Protein expression and degradation are fundamental to cell function and physiological status of organisms. Interfering with protein expression not only provides powerful strategies to analyze the function of proteins but also inspires effective treatment methods for diseases caused by protein dysfunction. Recently, harnessing the power of the ubiquitin-proteasome system for targeted protein degradation (TPD) has become the focus of researches. Over the past two decades, TPD technologies, such as E3 ligase modification, PROTACs, and the Trim-Away method, have successfully re-oriented the ubiquitin-proteasome pathway and thus degraded many pathogenic proteins and even "undruggable" targets. -

Overview of Antibody Drug Delivery
pharmaceutics Review Overview of Antibody Drug Delivery Sahar Awwad 1,2,* ID and Ukrit Angkawinitwong 1 1 UCL School of Pharmacy, London WC1N 1AX, UK; [email protected] 2 National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology, London EC1 V9EL, UK * Correspondence: [email protected]; Tel.: +44-207-753-5802 Received: 27 March 2018; Accepted: 29 June 2018; Published: 4 July 2018 Abstract: Monoclonal antibodies (mAbs) are one of the most important classes of therapeutic proteins, which are used to treat a wide number of diseases (e.g., oncology, inflammation and autoimmune diseases). Monoclonal antibody technologies are continuing to evolve to develop medicines with increasingly improved safety profiles, with the identification of new drug targets being one key barrier for new antibody development. There are many opportunities for developing antibody formulations for better patient compliance, cost savings and lifecycle management, e.g., subcutaneous formulations. However, mAb-based medicines also have limitations that impact their clinical use; the most prominent challenges are their short pharmacokinetic properties and stability issues during manufacturing, transport and storage that can lead to aggregation and protein denaturation. The development of long acting protein formulations must maintain protein stability and be able to deliver a large enough dose over a prolonged period. Many strategies are being pursued to improve the formulation and dosage forms of antibodies to improve efficacy and to increase the range of applications for the clinical use of mAbs. Keywords: antibodies; protein; pharmacokinetics; drug delivery; stability 1. -

Gold Levels Produced by Treatment with Auranofin and Sodium Aurothiomalate
Ann Rheum Dis: first published as 10.1136/ard.42.5.566 on 1 October 1983. Downloaded from Annals ofthe Rheumatic Diseases, 1983, 42, 566-570 Gold levels produced by treatment with auranofin and sodium aurothiomalate D. LEWIS,' H. A. CAPELL,1 C. J. McNEIL,2 M. S. IQBAL,2 D. H. BROWN, 2 AND W. E. SMITH2 From the 1CentreforRheumatic Diseases, University DepartmentofMedicine, Baird Street, Glasgow G4 OEH, and the 2Department ofPure and Applied Chemistry, University ofStrathclyde, Glasgow GJ IXL SUMMARY Sixty-three patients with rheumatoid arthritis were randomly divided into 3 groups, and treated with either sodium aurothiomalate (Myocrisin), auranofin, or placebo. Gold levels in whole blood, plasma, and haemolysate were measured serially along with clinical and laboratory para- meters of efficacy. Auranofin produced a higher ratio of haemolysate to plasma gold than Myocrisin, and it appears that the affinity of the red cell for gold is reduced during therapy with auranofin. Gold levels did not correlate with changes in the pain score, erythrocyte sedimentation rate, and C-reactive protein, nor with the development of toxicity. In the Myocrisin group the haemolysate gold level achieved was dependent on the number of cigarettes smoked. In the auranofin group there was no such correlation, but the haemolysate gold level was higher for smokers than non-smokers. The likely action of gold is discussed. copyright. Gold compounds have been used in the treatment of The purpose of the present study was to measure rheumatoid arthritis for over 50 years, and theirplace the distribution ofgold in the blood of patients receiv- in clinical practice is firmly established.' 2 However, ing Myocrisin or auranofin, both in order to deter- there remains a need to monitor the use of these mine whether or not parameters such as haemolysate compounds very carefully to establish efficacy and to gold or the ratio of haemolysate to plasma gold are anticipate the onset of any toxic reaction which may correlated with efficacy or toxicity and to improve http://ard.bmj.com/ occur. -

COVID-19: Mechanisms of Vaccination and Immunity
Review COVID-19: Mechanisms of Vaccination and Immunity Daniel E. Speiser 1,* and Martin F. Bachmann 2,3,4,* 1 Department of Oncology, University Hospital and University of Lausanne, 1066 Lausanne, Switzerland 2 International Immunology Centre, Anhui Agricultural University, Hefei 230036, China 3 Department of Rheumatology, Immunology and Allergology, Inselspital, University of Bern, 3010 Bern, Switzerland 4 Department of BioMedical Research, University of Bern, 3008 Bern, Switzerland * Correspondence: [email protected] (D.E.S.); [email protected] (M.F.B.) Received: 2 July 2020; Accepted: 20 July 2020; Published: 22 July 2020 Abstract: Vaccines are needed to protect from SARS-CoV-2, the virus causing COVID-19. Vaccines that induce large quantities of high affinity virus-neutralizing antibodies may optimally prevent infection and avoid unfavorable effects. Vaccination trials require precise clinical management, complemented with detailed evaluation of safety and immune responses. Here, we review the pros and cons of available vaccine platforms and options to accelerate vaccine development towards the safe immunization of the world’s population against SARS-CoV-2. Favorable vaccines, used in well-designed vaccination strategies, may be critical for limiting harm and promoting trust and a long-term return to normal public life and economy. Keywords: SARS-CoV-2; COVID-19; nucleic acid tests; serology; vaccination; immunity 1. Introduction The COVID-19 pandemic holds great challenges for which the world is only partially prepared [1]. SARS-CoV-2 combines serious pathogenicity with high infectivity. The latter is enhanced by the fact that asymptomatic and pre-symptomatic individuals can transmit the virus, in contrast to SARS-CoV-1 and MERS-CoV, which were transmitted by symptomatic patients and could be contained more efficiently [2,3]. -

Incubation Period of Measles from Exposure to Prodrome ■ Exposure to Rash Onset Averages 11 to 12 Days
Measles Paul Gastanaduy, MD; Penina Haber, MPH; Paul A. Rota, PhD; and Manisha Patel, MD, MS Measles is an acute, viral, infectious disease. References to measles can be found from as early as the 7th century. The Measles disease was described by the Persian physician Rhazes in the ● Acute viral infectious disease 10th century as “more to be dreaded than smallpox.” ● First described in 7th century In 1846, Peter Panum described the incubation period of ● Vaccines first licensed include measles and lifelong immunity after recovery from the disease. measles in 1963, MMR in 1971, John Enders and Thomas Chalmers Peebles isolated the virus in and MMRV in 2005 human and monkey kidney tissue culture in 1954. The first live, ● Infection nearly universal attenuated vaccine (Edmonston B strain) was licensed for use in during childhood in the United States in 1963. In 1971, a combined measles, mumps, prevaccine era and rubella (MMR) vaccine was licensed for use in the United ● Still common and often fatal States. In 2005, a combination measles, mumps, rubella, and in developing countries varicella (MMRV) vaccine was licensed. Before a vaccine was available, infection with measles virus was nearly universal during childhood, and more than 90% of persons were immune due to past infection by age 15 years. Measles is still a common and often fatal disease in developing countries. The World Health Organization estimates there were 142,300 deaths from measles globally in 2018. In the United 13 States, there have been recent outbreaks; the largest occurring in 2019 primarily among people who were not vaccinated. -

Hydroxychloroquine and Sulphasalazine Alone and Ann Rheum Dis: First Published As 10.1136/Ard.52.10.711 on 1 October 1993
Annals of the Rheumatic Diseases 1993; 52: 711-715 71 Hydroxychloroquine and sulphasalazine alone and Ann Rheum Dis: first published as 10.1136/ard.52.10.711 on 1 October 1993. Downloaded from in combination in rheumatoid arthritis: a randomised double blind trial Karen Lisbeth Faarvang, Charlotte Egsmose, Peter Kryger, Jan P0denphant, Margrethe Ingeman-Nielsen, Troels M0rk Hansen Abstract and hydroxychloroquine was superior to Objectives-To compare the effects of treatment with a single drug. The two drugs hydroxychloroquine and sulphasalazine were chosen because of their low incidence of alone and in combination in rheumatoid serious side effects. In addition, we aimed to arthritis. determine whether previous treatment with Methods-A six month randomised, gold salts or penicillamine affected the multicentre, double blind trial with three outcome. Finally, the selection of patients for parallel groups was performed. Ninety one the study from the total population of patients outpatients with active rheumatoid with RA at the three participating rheumato- arthritis were included. Monthly assess- logical clinics was recorded. ments of erythrocyte sedimentation rate, morning stiffness, number of swollen joints, a pain score, and global assess- Patients and methods ments were carried out. Radiographs of PATIENTS hands and wrists were taken before and During the inclusion period 776 patients with after the trial. RA were registered in the three participating Results-Sixty two patients completed the departments. Ninety one of these met the study. The 29 withdrawals caused no criteria for inclusion in the trial. They all had evident bias, and there was no difference RA defined according to the American in side effects among the three groups.