Defined Man's Origin As the Point at Which an Ape-Like Creature First Mad
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Homo Habilis
COMMENT SUSTAINABILITY Citizens and POLICY End the bureaucracy THEATRE Shakespeare’s ENVIRONMENT James Lovelock businesses must track that is holding back science world was steeped in on surprisingly optimistic governments’ progress p.33 in India p.36 practical discovery p.39 form p.41 The foot of the apeman that palaeo ‘handy man’, anthropologists had been Homo habilis. recovering in southern Africa since the 1920s. This, the thinking went, was replaced by the taller, larger-brained Homo erectus from Asia, which spread to Europe and evolved into Nean derthals, which evolved into Homo sapiens. But what lay between the australopiths and H. erectus, the first known human? BETTING ON AFRICA Until the 1960s, H. erectus had been found only in Asia. But when primitive stone-chop LIBRARY PICTURE EVANS MUSEUM/MARY HISTORY NATURAL ping tools were uncovered at Olduvai Gorge in Tanzania, Leakey became convinced that this is where he would find the earliest stone- tool makers, who he assumed would belong to our genus. Maybe, like the australopiths, our human ancestors also originated in Africa. In 1931, Leakey began intensive prospect ing and excavation at Olduvai Gorge, 33 years before he announced the new human species. Now tourists travel to Olduvai on paved roads in air-conditioned buses; in the 1930s in the rainy season, the journey from Nairobi could take weeks. The ravines at Olduvai offered unparalleled access to ancient strata, but field work was no picnic in the park. Water was often scarce. Leakey and his team had to learn to share Olduvai with all of the wild animals that lived there, lions included. -
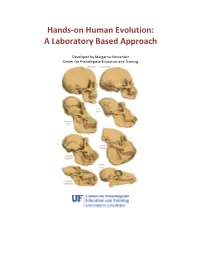
Hands-On Human Evolution: a Laboratory Based Approach
Hands-on Human Evolution: A Laboratory Based Approach Developed by Margarita Hernandez Center for Precollegiate Education and Training Author: Margarita Hernandez Curriculum Team: Julie Bokor, Sven Engling A huge thank you to….. Contents: 4. Author’s note 5. Introduction 6. Tips about the curriculum 8. Lesson Summaries 9. Lesson Sequencing Guide 10. Vocabulary 11. Next Generation Sunshine State Standards- Science 12. Background information 13. Lessons 122. Resources 123. Content Assessment 129. Content Area Expert Evaluation 131. Teacher Feedback Form 134. Student Feedback Form Lesson 1: Hominid Evolution Lab 19. Lesson 1 . Student Lab Pages . Student Lab Key . Human Evolution Phylogeny . Lab Station Numbers . Skeletal Pictures Lesson 2: Chromosomal Comparison Lab 48. Lesson 2 . Student Activity Pages . Student Lab Key Lesson 3: Naledi Jigsaw 77. Lesson 3 Author’s note Introduction Page The validity and importance of the theory of biological evolution runs strong throughout the topic of biology. Evolution serves as a foundation to many biological concepts by tying together the different tenants of biology, like ecology, anatomy, genetics, zoology, and taxonomy. It is for this reason that evolution plays a prominent role in the state and national standards and deserves thorough coverage in a classroom. A prime example of evolution can be seen in our own ancestral history, and this unit provides students with an excellent opportunity to consider the multiple lines of evidence that support hominid evolution. By allowing students the chance to uncover the supporting evidence for evolution themselves, they discover the ways the theory of evolution is supported by multiple sources. It is our hope that the opportunity to handle our ancestors’ bone casts and examine real molecular data, in an inquiry based environment, will pique the interest of students, ultimately leading them to conclude that the evidence they have gathered thoroughly supports the theory of evolution. -

Jane Goodall: a Timeline 3
Discussion Guide Table of Contents The Life of Jane Goodall: A Timeline 3 Growing Up: Jane Goodall’s Mission Starts Early 5 Louis Leakey and the ‘Trimates’ 7 Getting Started at Gombe 9 The Gombe Community 10 A Family of Her Own 12 A Lifelong Mission 14 Women in the Biological Sciences Today 17 Jane Goodall, in Her Own Words 18 Additional Resources for Further Study 19 © 2017 NGC Network US, LLC and NGC Network International, LLC. All rights reserved. 2 Journeys in Film : JANE The Life of Jane Goodall: A Timeline April 3, 1934 Valerie Jane Morris-Goodall is born in London, England. 1952 Jane graduates from secondary school, attends secretarial school, and gets a job at Oxford University. 1957 At the invitation of a school friend, Jane sails to Kenya, meets Dr. Louis Leakey, and takes a job as his secretary. 1960 Jane begins her observations of the chimpanzees at what was then Gombe Stream Game Reserve, taking careful notes. Her mother is her companion from July to November. 1961 The chimpanzee Jane has named David Greybeard accepts her, leading to her acceptance by the other chimpanzees. 1962 Jane goes to Cambridge University to pursue a doctorate, despite not having any undergraduate college degree. After the first term, she returns to Africa to continue her study of the chimpanzees. She continues to travel back and forth between Cambridge and Gombe for several years. Baron Hugo van Lawick, a photographer for National Geographic, begins taking photos and films at Gombe. 1964 Jane and Hugo marry in England and return to Gombe. -

Kenyan Stone Age: the Louis Leakey Collection
World Archaeology at the Pitt Rivers Museum: A Characterization edited by Dan Hicks and Alice Stevenson, Archaeopress 2013, pages 35-21 3 Kenyan Stone Age: the Louis Leakey Collection Ceri Shipton Access 3.1 Introduction Louis Seymour Bazett Leakey is considered to be the founding father of palaeoanthropology, and his donation of some 6,747 artefacts from several Kenyan sites to the Pitt Rivers Museum (PRM) make his one of the largest collections in the Museum. Leakey was passionate aboutopen human evolution and Africa, and was able to prove that the deep roots of human ancestry lay in his native east Africa. At Olduvai Gorge, Tanzania he excavated an extraordinary sequence of Pleistocene human evolution, discovering several hominin species and naming the earliest known human culture: the Oldowan. At Olorgesailie, Kenya, he excavated an Acheulean site that is still influential in our understanding of Lower Pleistocene human behaviour. On Rusinga Island in Lake Victoria, Kenya he found the Miocene ape ancestor Proconsul. He obtained funding to establish three of the most influential primatologists in their field, dubbed Leakey’s ‘ape women’; Jane Goodall, Dian Fossey and Birute Galdikas, who pioneered the study of chimpanzee, gorilla and orangutan behaviour respectively. His second wife Mary Leakey, whom he first hired as an artefact illustrator, went on to be a great researcher in her own right, surpassing Louis’ work with her own excavations at Olduvai Gorge. Mary and Louis’ son Richard followed his parents’ career path initially, discovering many of the most important hominin fossils including KNM WT 15000 (the Nariokotome boy, a near complete Homo ergaster skeleton), KNM WT 17000 (the type specimen for Paranthropus aethiopicus), and KNM ER 1470 (the type specimen for Homo rudolfensis with an extremely well preserved Archaeopressendocranium). -

Reconstructing Human Evolution: Achievements, Challenges, and Opportunities
Reconstructing human evolution: Achievements, challenges, and opportunities Bernard Wood1 George Washington University, Washington, DC 20052 This contribution reviews the evidence that has resolved the can then be used as the equivalent of a null hypothesis when branching structure of the higher primate part of the tree of life considering where to place newly discovered fossil great ape taxa. and the substantial body of fossil evidence for human evolution. It considers some of the problems faced by those who try to interpret The Human Fossil Record. The fossil record of the human clade the taxonomy and systematics of the human fossil record. How do consists of fossil evidence for modern humans plus that of all ex- you to tell an early human taxon from one in a closely related clade? tinct taxa that are hypothesized to be more closely related to How do you determine the number of taxa represented in the modern humans than to any other living taxon. Not so long ago human clade? How can homoplasy be recognized and factored into nearly all researchers were comfortable with according the human attempts to recover phylogeny? clade the status of a family, the Hominidae, with the nonhuman extant great apes (i.e., chimpanzees, bonobos, gorillas, and history | hominin orangutans) placed in a separate family, the Pongidae. But given the abundant evidence for a closer relationship between Pan and his contribution begins by considering two achievements rele- Homo than between Pan and Gorilla (see above), many research- Tvant to reconstructing human evolution: resolving the branch- ers have concluded that the human clade should be distinguished ing structure of the higher primate part of the tree of life and the beneath the level of the family in the Linnaean hierarchy. -

Dian Fossey's Gorillas 50 Years On
Dian Fossey’s gorillas 50 years on: research, conservation, and lessons learned Stacy Rosenbaum Institute for Mind and Biology University of Chicago An Outline 1. A brief history (natural and human) 2. The mission today àProtect, educate, develop, learn 3. My role: ongoing research 4. Successes, lessons learned, and why it all matters Two gorilla species: western & eastern Uganda Democratic Republic of Congo Rwanda KARISOKE History of mountain gorillas in western science • 1901- Western science ‘discovers’ mountain gorillas • 1920s - Carl Akeley expeditions lead to Albert National Park • 1960 - George Schaller writes first scientific articles • 1967 – Dian Fossey establishes Karisoke Research Center • 2016 – 70+ scientists contributed to knowledge of behavior, ecology 1963: Dian Fossey meets renowned anthropologist Dr. Louis Leakey She encounters mountain gorillas for the first time, and persuades Leakey to hire her Karisoke Research Center 1967-ongoing… Observing behavior • Habituated groups for study • Fossey learned to observe individuals KarisokeKarisoke Research Research Center Center 1967-ongoing…1967-ongoing… Karisoke’s central objectives • Protection • Education • Community Development • Research* Protection & conservation historically Protection & conservation today Illegal activities Habitat Loss Direct poaching Disease transmission Gorilla protection & monitoring • Protection and monitoring for habituated gorilla groups • Assistance to national park authorities Democratic Republic Uganda of Congo Rwanda Education Sites of engagement: • Primary classrooms • Zoos (USA) • Social media • National University of Rwanda “Citizen science” project Community development Combating poverty = improved conservation outcomes Hospital building Toilet facilities Water tanks Treating intestinal parasites Solar generators Gorilla Research Program Key Karisoke research findings • Gorillas aren’t King Kong! • Socioecological principles • Male and female dispersal • Population census Ongoing project #1: Mountain gorilla stress physiology Stress: causes & consequences Drs. -

Abbey, Cherie D., Ed. Biography Today: Scientists
DOCUMENT RESUME ED 423 192 SO 028 991 AUTHOR Harris, Laurie Lanzen, Ed.; Abbey, Cherie D., Ed. TITLE Biography Today: Scientists & Inventors Series. Profiles of People of Interest to Young Readers. Vol. 1, 1996. ISBN ISBN-0-7808-0068-2 PUB DATE 1996-00-00 NOTE 192p. AVAILABLE FROM Omnigraphics, Inc., 2500 Penobscot Building, Detroit, MI 48226. PUB TYPE Books (010) EDRS PRICE MF01/PC08 Plus Postage. DESCRIPTORS Biographies; *Childrens Literature; *Current Events; Elementary Secondary Education; *Inventions; Popular Culture; Profiles; Recreational Reading; Reference Materials; *Role Models; *Scientists; Student Interests; Supplementary Reading Materials ABSTRACT This issue of "Biography Today" looks at scientists and inventors and is created to appeal to young readers in a format they can and enjoy and easily understand. Each entry provides at least one picture of the individual profiled, and bold-faced rubrics lead the reader to information on birth, youth, early memories, education, first jobs, marriage and family, career highlights, memorable experiences, hobbies, and honors and awards. Entries also provide information on further reading for readers. Obituary entries are included to provide a perspective on an individual's entire career. Each issue concludes with a name index, a general index, a birthplace index, and a birthday index. The scientists and inventors highlighted are John Bardeen (obituary), Sylvia Earle, Dian Fossey (obituary), Jane Goodall, Bernadine Healy, Jack Horner, Mathilde Krim, Edwin Land (obituary), Louis Leakey, Mary Leakey, Rita Levi-Montalcini, J. Robert Oppenheimer (obituary), Albert Sabin,(obituary), Carl Sagan, and James D. Watson. (RJC) ******************************************************************************** Reproductions supplied by EDRS are the best that can be made from the original document. -

Louis Leakey and Mary Leakey the First Family of Palaeoanthropology
GENERAL ¨ ARTICLE Louis Leakey and Mary Leakey The First Family of Palaeoanthropology Rajan Gaur Louis and Mary Leakey are two famous palaeoanthropologists and archaeologists of the twentieth century whose discoveries have had a major influence on our understanding of human evolution. These discoveries were spectacular and brought popular attention to the field of palaeoanthropology, though at times they also courted controversies. Though, debate on the proper interpretation of many of the fossil hominid finds Rajan Gaur is a Professor made by them continues even today, no one questions their at the Department of tremendous contribution to our knowledge about evolution of Anthropology, Panjab humankind. This article is focussed on their life and works. University,Chandigarh. His major area of research has been Introduction palaeoanthropology, mammalian palaeontology, Ever since Darwin published his monumental work in his book palaeoecology and physical ‘On the Origin of Species’ in 1859, the idea of evolution has anthropology. Over the gradually caught the imagination of the scientific community. last thirtyyears he has led However, it took a while for the concept of human evolution to be several palaeoanthropological accepted, in the face of the millennia-old and deeply entrenched fieldworks in the Siwaliks biblical view of the universe as a static representation of God’s of Northwest India and the creative device. Before Darwin’s theory, most scholars believed Narmada Basin and the explanation of Archbishop James Ussher of Armagh, Ireland collected a number of vertebrate fossils. and John Lightfoot (Vice Chancellor of the University of Cam- bridge), who in the early 1600s had deduced from a careful study of the Bible that earth had been created at 9 o’clock on October 23, 4004 BC. -

Kiko Record on Appeal (Vol. 2 of 2)
New York County Clerk’s Index No. 150149/16 New York Supreme Court APPELLATE DIVISION — FIRST DEPARTMENT >> >> In the Matter of a Proceeding under Article 70 of the CPLR for a Writ of Habeas Corpus, THE NONHUMAN RIGHTS PROJECT, INC., on behalf of KIKO, Petitioner-Appellant, against CARMEN PRESTI, individually and as an officer and director of The Primate Sanctuary, Inc., CHRISTIE E. PRESTI, individually and as an officer and director of The Primate Sanctuary, Inc., and THE PRIMATE SANCTUARY INC., Respondents. RECORD ON APPEAL VOLUME II OF II Pages 394 to 780 CARMEN PRESTI, ELIZABETH STEIN, ESQ. individually and as an officer 5 Dunhill Road and director of The Primate New Hyde Park, New York 11040 Sanctuary Inc. 516-747-4726 2764 Livingston Avenue [email protected] Niagara Falls, New York 14303 and 716-284-6118 [email protected] STEVEN M. WISE, ESQ. (of the bar of the State of Respondent Pro Se Massachusetts) by permission of the Court CHRISTIE E. PRESTI individually and as an officer 5195 NW 112th Terrace and director of The Primate Coral Springs, Florida 33076 Sanctuary Inc. 954-648-9864 2764 Livingston Avenue [email protected] Niagara Falls, New York 14303 Attorneys for Petitioner-Appellant 716-284-6118 [email protected] Respondent Pro Se (Additional Counsel Continued Inside) Printed on Recycled Paper THE PRIMATE SANTUARY INC. 2764 Livingston Avenue Niagara Falls, New York 14303 716-284-6118 [email protected] Respondent Pro Se Table of Contents Page Volume I Pre-Argument Statement .......................................................................... 1 Notice of Appeal, dated February 9, 2016 ................................................ 5 Declined Order to Show Cause and Memorandum of the Honorable Barbara Jaffe, Appealed From ........................................ -

Confessions of a Biologist African Ancestors
_7~---------------------------------------S~NGBCXJKS;------------------------N_A_ru_~__ v_oL_._n __ ~_~ __ R,_L_,~_ period during which Italy and Europe The remainder of the book is about Confessions of a underwent cataclysmic political and social Luria's interest in literature, especially changes. I would have liked to have heard poetry, his commitment to politics and his biologist more about this time of his life; given his emotional development, each considered Sydney Brenner avowed existentialist views, he should have separately, as examples of his sectorial told us more about the formative years theory of autobiography. A Slot Machine, A Broken Test Tube: instead of merely flitting through them. I found the book disappointing. An Autobiography. By S.E. Luria. Indeed, the remainder ofthe historical nar Although not a contemporary, I know Harper& Row: 1984. Pp.228. $17.95. To rative is treated in the same brief manner, many of the protagonists and have lived be published in the UK on and is like an abstract of an autobiography through many of the events in science; I 31 May, £12.50. rather than the book itself. also know Luria and over the years have The middle part, "The Science Path", naturally formed my own opinion of him. describes his scientific work and develop Perhaps, like writers of autobiographies, AN autobiography allows a man the oppor ment. All elderly molecular geneticists reviewers need to be open and divulge their tunity to tell all about his life and work, to know of the Luria-Delbrilck fluctuation private judgements. There is a sense in reveal his secret motives and ambitions, experiment which proved which this book might be and to give a personal view of the world and that mutants pre-exist in even more self-revealing the people he has known in it. -

CLE Reading Materials
ANIMAL PROPERTY RIGHTS KAREN BRADSHAW* The animal rights movement largely focuses on protecting species whose suffering is most visible to humans, such as pets, livestock, and captive mammals. Yet, we do not observe how unsustainable land development and fishing practices are harming many species of wildlife and sea creatures. Fish and wildlife populations have recently suffered staggering losses, and they stand to lose far more. This Article proposes a new legal approach to protect these currently overlooked creatures. I suggest extending property rights to animals, which would allow them to own land, water, and natural resources. Human trustees would manage animal-owned trusts managed at the ecosystem level—a structure that fits within existing legal institutions. Although admittedly radical, an animal property rights regime would create tremendous gains for imperiled species with relatively few costs to humans. INTRODUCTION .......................................................................... 810 I. POLARIZED ANIMAL LAW ................................................... 817 II. GRANTING ANIMALS PROPERTY RIGHTS ........................... 823 III. IMPLEMENTING AN ANIMAL PROPERTY RIGHTS REGIME.. 830 A. Statutory Approach ................................................... 831 1. Ownership Structures ......................................... 831 2. Management ........................................................ 835 * Associate Professor of Law, Sandra Day O’Connor College of Law, Arizona State University; Sustainability Scholar, -

6 Lucy & the Leakeys
6 LUCY & THE LEAKEYS BIOGRAPHY 770L LUCY & THE LEAKEYS HOMININE FOSSILS AND PALEOARCHAEOLOGISTS Louis Leakey Mary Leakey Born Born August 7, 1903 February 6, 1913 Kabete, Kenya London, England Lucy Died Died c. 3.2 MYA October 1, 1972 December 9, 1996 Afar, Ethiopia London, England Nairobi, Kenya By Cynthia Stokes Brown, adapted by Newsela NO INTRO INCLUDED 2 3 same line. Then, about 5 to 7 million years ago, a new line split off from the Human origins chimpanzees. The new group lived in open savanna rather than in rain forest jungle. The old group in the rain forest continued to evolve. Two of its spe- Most scientists agree that humans emerged in Africa about 200,000 years cies still exist: the common chimpanzee and the bonobo. ago. Scientists believe this because fossilized bones and skulls were uncov- ered in East Africa. Ash from volcanoes was found near the bones. By test- The new group in the savanna evolved over thousands of years. If formed ing the ash, scientists could tell their ages. The bones and skulls range from into several species. It’s unclear how many, but we know of at least 18. Finally, 25,000 to 4.4 million years old. They show many different stages of human only one was left: humans, or Homo sapiens. All the species before us back and primate evolution. Paleoarchaeologists uncovered the fossils. These to our common ancestor with chimpanzees are now called “hominins.” They scientists study the remains of humans throughout evolution. used to be called “hominids.” Based on the fossil evidence, paleoarchaeologists tell this story: For 99.9 Try visualizing it like this.