Antibiotic Recommendations for Sepsis and Septic Shock
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Medical Review(S) Clinical Review
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 200327 MEDICAL REVIEW(S) CLINICAL REVIEW Application Type NDA Application Number(s) 200327 Priority or Standard Standard Submit Date(s) December 29, 2009 Received Date(s) December 30, 2009 PDUFA Goal Date October 30, 2010 Division / Office Division of Anti-Infective and Ophthalmology Products Office of Antimicrobial Products Reviewer Name(s) Ariel Ramirez Porcalla, MD, MPH Neil Rellosa, MD Review Completion October 29, 2010 Date Established Name Ceftaroline fosamil for injection (Proposed) Trade Name Teflaro Therapeutic Class Cephalosporin; ß-lactams Applicant Cerexa, Inc. Forest Laboratories, Inc. Formulation(s) 400 mg/vial and 600 mg/vial Intravenous Dosing Regimen 600 mg every 12 hours by IV infusion Indication(s) Acute Bacterial Skin and Skin Structure Infection (ABSSSI); Community-acquired Bacterial Pneumonia (CABP) Intended Population(s) Adults ≥ 18 years of age Template Version: March 6, 2009 Reference ID: 2857265 Clinical Review Ariel Ramirez Porcalla, MD, MPH Neil Rellosa, MD NDA 200327: Teflaro (ceftaroline fosamil) Table of Contents 1 RECOMMENDATIONS/RISK BENEFIT ASSESSMENT ......................................... 9 1.1 Recommendation on Regulatory Action ........................................................... 10 1.2 Risk Benefit Assessment.................................................................................. 10 1.3 Recommendations for Postmarketing Risk Evaluation and Mitigation Strategies ........................................................................................................................ -

Charm II Antibiotic Analysis—Grain
Charm ii Antibiotic Analysis for Grain Products PROCeDURAL FLOWCHART + Binding Tracer Reagent Tablet Tablet Sample Charm ii 7600 analyzer Incubate START STOP Centrifuge Families DeteCteD = Aminoglycosides = Amphenicols/Chloramphenicol Resuspend = Beta-lactams = Macrolides = Sulfonamides C2Soft = Tetracyclines (optional) Count Results SAMPLE SIZe 50 to 100 g Computer Report SAMPLE PREPARATION Homogenize product in extraction solution for 60 seconds. Filter or centrifuge for 3 minutes. sample printout Test supernatant. Date = 08/23/10 preparation time Approximately 10-15 minutes, Time = 14:28:12 depending on the number of Operator = 1 samples. Time Counted = 60 Sample I.D. = 7764 ASSAY TIME Approximately 10 minutes, depending on drug family. Assay = Chloramphenicol CAPACITY 6 to 12 samples in assay, Lot# = ATBL 014 depending on drug family. Control Point = 2564 Sample (CPM) = 3676 Interpretation = Not Found Charm sciences, inc. 659 Andover Street, Lawrence, MA 01843, USA | Tel: +1.978.687.9200 | www.charm.com Charm ii Antibiotic Analysis for Grain Products Charm ii Kit Drug test sensitivity 1 (ppb) aminoglycosides (STIIHG) Streptomycin 2000 Dihydrostreptomycin 7500 Gentamicin 5000 aminoglycosides (GIIHG) Gentamicin 1000 Neomycin 500 Beta-lactams (PIIG) Penicillin-G 200 Amoxicillin 450 Ampicillin 400 Cephapirin 200 Ceftiofur 500 Cloxacillin 2500 Oxacillin 3750 Dicloxacillin 2500 Cefazolin 1500 Cefodroxil 1500 Cefotaxime 400 Cephalexin 1500 Cephradine 1500 Cefquinome 1000 Hetacillin 400 Nafcillin 3000 Penethamate 200 Piperacillin 1000 Ticarcillin 3500 Chloramphenicol Chloramphenicol 40 Florfenicol 160 & other amphenicols (CIIHG) Thiamphenicol 200 Chloramphenicol (AIIHG) Chloramphenicol 5 macrolides (EIIG) Erythromycin 1000 Tylosin 1000 Spiramycin 1000 Pirlimycin 2000 Tilmicosin 1000 Lincomycin 2500 sulfonamides (SMIIHG) Sulfamethazine 500 Sulfadimethoxine 200 Sulfathiazole 400 Sulfadiazine 200 tetracyclines (TIIHG) Tetracycline 100 Chlortetracycline 800 Oxytetracycline 800 1 Exceed 90% positive at a 95% confidence limit Charm sciences, inc. -

Sepsis: a Guide for Patients & Relatives
SEPSIS: A GUIDE FOR PATIENTS & RELATIVES CONTENTS ABOUT SEPSIS ABOUT SEPSIS: INTRODUCTION P3 What is sepsis? In the UK, at least 150,000* people each year suffer from serious P4 Why does sepsis happen? sepsis. Worldwide it is thought that 3 in a 1000 people get sepsis P4 Different types of sepsis P4 Who is at risk of getting sepsis? each year, which means that 18 million people are affected. P5 What sepsis does to your body Sepsis can move from a mild illness to a serious one very quickly, TREATMENT OF SEPSIS which is very frightening for patients and their relatives. P7 Why did I need to go to the Critical Care Unit? This booklet is for patients and relatives and it explains sepsis P8 What treatment might I have had? and its causes, the treatment needed and what might help after P9 What other help might I have received in the Critical Care Unit? having sepsis. It has been written by the UK Sepsis Trust, a charity P10 How might I have felt in the Critical Care Unit? which supports people who have had sepsis and campaigns to P11 How long might I stay in the Critical Care Unit and hospital? raise awareness of the illness, in collaboration with ICU steps. P11 Moving to a general ward and The Outreach Team/Patient at Risk Team If a patient cannot read this booklet for him or herself, it may be helpful for AFTER SEPSIS relatives to read it. This will help them to understand what the patient is going through and they will be more able to support them as they recover. -

UNASYN® (Ampicillin Sodium/Sulbactam Sodium)
® UNASYN (ampicillin sodium/sulbactam sodium) To reduce the development of drug-resistant bacteria and maintain the effectiveness of UNASYN® and other antibacterial drugs, UNASYN should be used only to treat infections that are proven or strongly suspected to be caused by bacteria. DESCRIPTION UNASYN is an injectable antibacterial combination consisting of the semisynthetic antibacterial ampicillin sodium and the beta-lactamase inhibitor sulbactam sodium for intravenous and intramuscular administration. Ampicillin sodium is derived from the penicillin nucleus, 6-aminopenicillanic acid. Chemically, it is monosodium (2S, 5R, 6R)-6-[(R)-2-amino-2-phenylacetamido]-3, 3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate and has a molecular weight of 371.39. Its chemical formula is C16H18N3NaO4S. The structural formula is: COONa O CH 3 N CH O 3 NH S NH 2 1 Reference ID: 4053909 Sulbactam sodium is a derivative of the basic penicillin nucleus. Chemically, sulbactam sodium is sodium penicillinate sulfone; sodium (2S, 5R)-3,3-dimethyl-7-oxo-4-thia- 1-azabicyclo [3.2.0] heptane-2-carboxylate 4,4-dioxide. Its chemical formula is C8H10NNaO5S with a molecular weight of 255.22. The structural formula is: COONa CH 3 O N CH 3 S O O UNASYN, ampicillin sodium/sulbactam sodium parenteral combination, is available as a white to off-white dry powder for reconstitution. UNASYN dry powder is freely soluble in aqueous diluents to yield pale yellow to yellow solutions containing ampicillin sodium and sulbactam sodium equivalent to 250 mg ampicillin per mL and 125 mg sulbactam per mL. The pH of the solutions is between 8.0 and 10.0. -

Severe Sepsis and Septic Shock Antibiotic Guide
Stanford Health Issue Date: 05/2017 Stanford Antimicrobial Safety and Sustainability Program Severe Sepsis and Septic Shock Antibiotic Guide Table 1: Antibiotic selection options for healthcare associated and/or immunocompromised patients • Healthcare associated: intravenous therapy, wound care, or intravenous chemotherapy within the prior 30 days, residence in a nursing home or other long-term care facility, hospitalization in an acute care hospital for two or more days within the prior 90 days, attendance at a hospital or hemodialysis clinic within the prior 30 days • Immunocompromised: Receiving chemotherapy, known systemic cancer not in remission, ANC <500, severe cell-mediated immune deficiency Table 2: Antibiotic selection options for community acquired, immunocompetent patients Table 3: Antibiotic selection options for patients with simple sepsis, community acquired, immunocompetent patients requiring hospitalization. Risk Factors for Select Organisms P. aeruginosa MRSA Invasive Candidiasis VRE (and other resistant GNR) Community acquired: • Known colonization with MDROs • Central venous catheter • Liver transplant • Prior IV antibiotics within 90 day • Recent MRSA infection • Broad-spectrum antibiotics • Known colonization • Known colonization with MDROs • Known MRSA colonization • + 1 of the following risk factors: • Prolonged broad antibacterial • Skin & Skin Structure and/or IV access site: ♦ Parenteral nutrition therapy Hospital acquired: ♦ Purulence ♦ Dialysis • Prolonged profound • Prior IV antibiotics within 90 days ♦ Abscess -
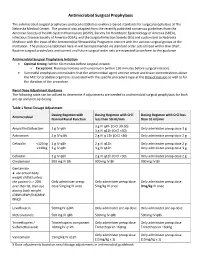
Antimicrobial Surgical Prophylaxis
Antimicrobial Surgical Prophylaxis The antimicrobial surgical prophylaxis protocol establishes evidence-based standards for surgical prophylaxis at The Nebraska Medical Center. The protocol was adapted from the recently published consensus guidelines from the American Society of Health-System Pharmacists (ASHP), Society for Healthcare Epidemiology of America (SHEA), Infectious Disease Society of America (IDSA), and the Surgical Infection Society (SIS) and customized to Nebraska Medicine with the input of the Antimicrobial Stewardship Program in concert with the various surgical groups at the institution. The protocol established here-in will be implemented via standard order sets utilized within One Chart. Routine surgical prophylaxis and current and future surgical order sets are expected to conform to this guidance. Antimicrobial Surgical Prophylaxis Initiation Optimal timing: Within 60 minutes before surgical incision o Exceptions: Fluoroquinolones and vancomycin (within 120 minutes before surgical incision) Successful prophylaxis necessitates that the antimicrobial agent achieve serum and tissue concentrations above the MIC for probable organisms associated with the specific procedure type at the time of incision as well as for the duration of the procedure. Renal Dose Adjustment Guidance The following table can be utilized to determine if adjustments are needed to antimicrobial surgical prophylaxis for both pre-op and post-op dosing. Table 1 Renal Dosage Adjustment Dosing Regimen with Dosing Regimen with CrCl Dosing Regimen with -

Septic Shock V9.0 Patient Flow Map
Septic Shock v9.0 Patient Flow Map Approval & Citation Summary of Version Changes Explanation of Evidence Ratings Patient presents to the ED with fever and/or concern for infection and ED sepsis score ≥ 6 ! BPA fires Use the ED Suspected Septic Shock RN and Well-appearing patients should be placed pathway for all ill on the appropriate ED CSW pathway for Provider appearing patients their underlying condition (e.g. ED No including HemOnc/BMT, Huddle: HemOnc BMT Suspected Infection, ED Central Line Infection Is the patient ill Suspected Central Line Infection, ED and Neonates appearing? Neonatal Fever) Yes ED Septic Shock Pathway • Use ED Suspected Septic Shock Plan • Antibiotics and blood cultures for specific populations included Inpatient Admit Criteria Does NOT meet Inpatient Admit Minute criteria • Resolution of hypotension and no • Admit to ICU ongoing signs of sepsis after ≤ 40 ml / 60 Huddle: YES NO • Follow ICU Septic Shock Pathway kg NS bolus Does patient meet • Use PICU/CICU Septic Shock Admit • First dose antibiotics administered Inpatient admit Plan • RISK to follow criteria? • Antibiotics, blood cultures for specific populations included in sub plans Previously healthy > 30 days RISK RN to follow all • Admit to General Medicine patients admitted with • Follow Admit from ED Septic Shock concern for sepsis Pathway • Use Inpatient Septic Shock Plan ! Concern for evolving sepsis Previously healthy < 30 days Any • Admit to General Medicine admitted • Call RRT or Code Blue • Follow Neonatal Fever Pathway patient with • Follow Inpatient -

Antimicrobial Stewardship Guidance
Antimicrobial Stewardship Guidance Federal Bureau of Prisons Clinical Practice Guidelines March 2013 Clinical guidelines are made available to the public for informational purposes only. The Federal Bureau of Prisons (BOP) does not warrant these guidelines for any other purpose, and assumes no responsibility for any injury or damage resulting from the reliance thereof. Proper medical practice necessitates that all cases are evaluated on an individual basis and that treatment decisions are patient-specific. Consult the BOP Clinical Practice Guidelines Web page to determine the date of the most recent update to this document: http://www.bop.gov/news/medresources.jsp Federal Bureau of Prisons Antimicrobial Stewardship Guidance Clinical Practice Guidelines March 2013 Table of Contents 1. Purpose ............................................................................................................................................. 3 2. Introduction ...................................................................................................................................... 3 3. Antimicrobial Stewardship in the BOP............................................................................................ 4 4. General Guidance for Diagnosis and Identifying Infection ............................................................. 5 Diagnosis of Specific Infections ........................................................................................................ 6 Upper Respiratory Infections (not otherwise specified) .............................................................................. -

Below Are the CLSI Breakpoints for Selected Bacteria. Please Use Your Clinical Judgement When Assessing Breakpoints
Below are the CLSI breakpoints for selected bacteria. Please use your clinical judgement when assessing breakpoints. The lowest number does NOT equal most potent antimicrobial. Contact Antimicrobial Stewardship for drug selection and dosing questions. Table 1: 2014 MIC Interpretive Standards for Enterobacteriaceae (includes E.coli, Klebsiella, Enterobacter, Citrobacter, Serratia and Proteus spp) Antimicrobial Agent MIC Interpretive Criteria (g/mL) Enterobacteriaceae S I R Ampicillin ≤ 8 16 ≥ 32 Ampicillin-sulbactam ≤ 8/4 16/8 ≥ 32/16 Aztreonam ≤ 4 8 ≥ 16 Cefazolin (blood) ≤ 2 4 ≥ 8 Cefazolin** (uncomplicated UTI only) ≤ 16 ≥ 32 Cefepime* ≤ 2 4-8* ≥ 16 Cefotetan ≤ 16 32 ≥ 64 Ceftaroline ≤ 0.5 1 ≥ 2 Ceftazidime ≤ 4 8 ≥ 16 Ceftriaxone ≤ 1 2 ≥ 4 Cefpodoxime ≤ 2 4 ≥ 8 Ciprofloxacin ≤ 1 2 ≥ 4 Ertapenem ≤ 0.5 1 ≥ 2 Fosfomycin ≤ 64 128 ≥256 Gentamicin ≤ 4 8 ≥ 16 Imipenem ≤ 1 2 ≥ 4 Levofloxacin ≤ 2 4 ≥ 8 Meropenem ≤ 1 2 ≥ 4 Piperacillin-tazobactam ≤ 16/4 32/4 – 64/4 ≥ 128/4 Trimethoprim-sulfamethoxazole ≤ 2/38 --- ≥ 4/76 *Susceptibile dose-dependent – see chart below **Cefazolin can predict results for cefaclor, cefdinir, cefpodoxime, cefprozil, cefuroxime axetil, cephalexin and loracarbef for uncomplicated UTIs due to E.coli, K.pneumoniae, and P.mirabilis. Cefpodoxime, cefinidir, and cefuroxime axetil may be tested individually because some isolated may be susceptible to these agents while testing resistant to cefazolin. Cefepime dosing for Enterobacteriaceae ( E.coli, Klebsiella, Enterobacter, Citrobacter, Serratia & Proteus spp) Susceptible Susceptible –dose-dependent (SDD) Resistant MIC </= 2 4 8 >/= 16 Based on dose of: 1g q12h 1g every 8h or 2g every 8 h Do not give 2g q12 Total dose 2g 3-4g 6g NA Table 2: 2014 MIC Interpretive Standards for Pseudomonas aeruginosa and Acinetobacter spp. -

Summary of Product Characteristics
SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Augmentin 125 mg/31.25 mg/5 ml powder for oral suspension Augmentin 250 mg/62.5 mg/5 ml powder for oral suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION When reconstituted, every ml of oral suspension contains amoxicillin trihydrate equivalent to 25 mg amoxicillin and potassium clavulanate equivalent to 6.25 mg of clavulanic acid. Excipients with known effect Every ml of oral suspension contains 2.5 mg aspartame (E951). The flavouring in Augmentin contains maltodextrin (glucose) (see section 4.4). This medicine contains less than 1 mmol sodium (23 mg) per ml, that is to say essentially ‘sodium- free’. When reconstituted, every ml of oral suspension contains amoxicillin trihydrate equivalent to 50 mg amoxicillin and potassium clavulanate equivalent to 12.5 mg of clavulanic acid. Excipients with known effect Every ml of oral suspension contains 2.5 mg aspartame (E951). The flavouring in Augmentin contains maltodextrin (glucose) (see section 4.4). This medicine contains less than 1 mmol sodium (23 mg) per ml, that is to say essentially ‘sodium- free’. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Powder for oral suspension. Off-white powder. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Augmentin is indicated for the treatment of the following infections in adults and children (see sections 4.2, 4.4 and 5.1): • Acute bacterial sinusitis (adequately diagnosed) • Acute otitis media • Acute exacerbations of chronic bronchitis (adequately diagnosed) • Community acquired pneumonia • Cystitis • Pyelonephritis 2 • Skin and soft tissue infections in particular cellulitis, animal bites, severe dental abscess with spreading cellulitis • Bone and joint infections, in particular osteomyelitis. -

Pneumonia in the Context of Severe Sepsis: a Significant Diagnostic Problem
-1 plasma D-dimer level was low (282 mg?L ). Chest radiography affected by antiphospholipid antibodies syndrome and showed multiple ill-defined increased densities in both lower avoided diagnostic surgery. lung fields. High-resolution computed tomography was then In conclusion, we believe that the indications for use of performed and showed ill-defined, wedge-shaped increased endobronchial ultrasound are manifold and are certainly densities with patent airways in the posterior subpleural greater than those so far recognised. This procedure should regions of both lower lobes. The computed tomography therefore be implemented and further developed in interven- differential diagnosis was organising pneumonia, bronchop- tional pneumology. neumonia, Churg–Strauss syndrome, Wegener granulomato- sis, pulmonary haemorrhage, or vasculitis of various causes. Transbronchial lung biopsy was performed in the right lower G.L. Casoni, C. Gurioli, M. Romagnoli and V. Poletti lobe. Microscopic examination showed alveolar haemorrhage Thoracic Dept, G.B. Morgagni Hospital, Forlı´, Italy. without evidence of vasculitis, eosinophilic infiltration or organising pneumonia. Serum circulating antiphospholipid STATEMENT OF INTEREST antibodies were eventually found. Therefore, pulmonary None declared. angiography was performed and an intra-arterial low density was found in the right main pulmonary artery. This finding SUPPLEMENTARY MATERIAL was believed to be compatible with pulmonary thrombo- This article has supplementary material accessible from embolism; however, it didn’t exclude a possible right www.erj.ersjournals.com pulmonary artery sarcoma. In this case, the only procedure that would rule out the presence of a right pulmonary artery sarcoma (without delays in diagnosis) with any reasonable REFERENCES certainty was surgery. 1 Herth F, Becker HD, LoCicero J 3rd, Ernst A., Endobronchial We decided to perform rigid bronchoscopy under general ultrasound in therapeutic bronchoscopy. -

Penicillin Allergy Guidance Document
Penicillin Allergy Guidance Document Key Points Background Careful evaluation of antibiotic allergy and prior tolerance history is essential to providing optimal treatment The true incidence of penicillin hypersensitivity amongst patients in the United States is less than 1% Alterations in antibiotic prescribing due to reported penicillin allergy has been shown to result in higher costs, increased risk of antibiotic resistance, and worse patient outcomes Cross-reactivity between truly penicillin allergic patients and later generation cephalosporins and/or carbapenems is rare Evaluation of Penicillin Allergy Obtain a detailed history of allergic reaction Classify the type and severity of the reaction paying particular attention to any IgE-mediated reactions (e.g., anaphylaxis, hives, angioedema, etc.) (Table 1) Evaluate prior tolerance of beta-lactam antibiotics utilizing patient interview or the electronic medical record Recommendations for Challenging Penicillin Allergic Patients See Figure 1 Follow-Up Document tolerance or intolerance in the patient’s allergy history Consider referring to allergy clinic for skin testing Created July 2017 by Macey Wolfe, PharmD; John Schoen, PharmD, BCPS; Scott Bergman, PharmD, BCPS; Sara May, MD; and Trevor Van Schooneveld, MD, FACP Disclaimer: This resource is intended for non-commercial educational and quality improvement purposes. Outside entities may utilize for these purposes, but must acknowledge the source. The guidance is intended to assist practitioners in managing a clinical situation but is not mandatory. The interprofessional group of authors have made considerable efforts to ensure the information upon which they are based is accurate and up to date. Any treatments have some inherent risk. Recommendations are meant to improve quality of patient care yet should not replace clinical judgment.