Neurontin (Gabapentin)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neurontin (Gabapentin)
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Gabapentin Clinical Criteria Information Included in this Document Neurontin (gabapentin) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. Gralise (gabapentin Extended Release) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. March 29, 2019 Copyright © 2019 Health Information Designs, LLC 1 Horizant -

Clomipramine | Memorial Sloan Kettering Cancer Center
PATIENT & CAREGIVER EDUCATION Clomipramine This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US Anafranil Brand Names: Canada Anafranil; MED ClomiPRAMINE; TARO-Clomipramine Warning Drugs like this one have raised the chance of suicidal thoughts or actions in children and young adults. The risk may be greater in people who have had these thoughts or actions in the past. All people who take this drug need to be watched closely. Call the doctor right away if signs like low mood (depression), nervousness, restlessness, grouchiness, panic attacks, or changes in mood or actions are new or worse. Call the doctor right away if any thoughts or actions of suicide occur. This drug is not approved for use in all children. Talk with the doctor to be sure that this drug is right for your child. What is this drug used for? It is used to treat obsessive-compulsive problems. It may be given to you for other reasons. Talk with the doctor. Clomipramine 1/8 What do I need to tell my doctor BEFORE I take this drug? If you have an allergy to clomipramine or any other part of this drug. If you are allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had. If you have had a recent heart attack. If you are taking any of these drugs: Linezolid or methylene blue. -

Big Pain Assays Aren't a Big Pain with the Raptor Biphenyl LC Column
Featured Application: 231 Pain Management and Drugs of Abuse Compounds in under 10 Minutes by LC-MS/MS Big Pain Assays Aren’t a Big Pain with the Raptor Biphenyl LC Column • 231 compounds, 40+ isobars, 10 drug classes, 22 ESI- compounds in 10 minutes with 1 column. • A Raptor SPP LC column with time-tested Restek Biphenyl selectivity is the most versatile, multiclass-capable LC column available. • Achieve excellent separation of critical isobars with no tailing peaks. • Run fast and reliable high-throughput LC-MS/MS analyses with increased sensitivity using simple mobile phases. The use of pain management drugs is steadily increasing. As a result, hospital and reference labs are seeing an increase in patient samples that must be screened for a wide variety of pain management drugs to prevent drug abuse and to ensure patient safety and adherence to their medication regimen. Thera- peutic drug monitoring can be challenging due to the low cutoff levels, potential matrix interferences, and isobaric drug compounds. To address these chal- lenges, many drug testing facilities are turning to liquid chromatography coupled with mass spectrometry (LC-MS/MS) for its increased speed, sensitivity, and specificity. As shown in the analysis below, Restek’s Raptor Biphenyl column is ideal for developing successful LC-MS/MS pain medication screening methodologies. With its exceptionally high retention and unique selectivity, 231 multiclass drug compounds and metabolites—including over 40 isobars—can be analyzed in just 10 minutes. In addition, separate panels have been optimized on the Raptor Biphenyl column specifically for opioids, antianxiety drugs, barbiturates, NSAIDs and analgesics, antidepressants, antiepileptics, antipsychotics, hallucinogens, and stimulants for use during confirmation and quantitative analyses. -

Chapter 25 Mechanisms of Action of Antiepileptic Drugs
Chapter 25 Mechanisms of action of antiepileptic drugs GRAEME J. SILLS Department of Molecular and Clinical Pharmacology, University of Liverpool _________________________________________________________________________ Introduction The serendipitous discovery of the anticonvulsant properties of phenobarbital in 1912 marked the foundation of the modern pharmacotherapy of epilepsy. The subsequent 70 years saw the introduction of phenytoin, ethosuximide, carbamazepine, sodium valproate and a range of benzodiazepines. Collectively, these compounds have come to be regarded as the ‘established’ antiepileptic drugs (AEDs). A concerted period of development of drugs for epilepsy throughout the 1980s and 1990s has resulted (to date) in 16 new agents being licensed as add-on treatment for difficult-to-control adult and/or paediatric epilepsy, with some becoming available as monotherapy for newly diagnosed patients. Together, these have become known as the ‘modern’ AEDs. Throughout this period of unprecedented drug development, there have also been considerable advances in our understanding of how antiepileptic agents exert their effects at the cellular level. AEDs are neither preventive nor curative and are employed solely as a means of controlling symptoms (i.e. suppression of seizures). Recurrent seizure activity is the manifestation of an intermittent and excessive hyperexcitability of the nervous system and, while the pharmacological minutiae of currently marketed AEDs remain to be completely unravelled, these agents essentially redress the balance between neuronal excitation and inhibition. Three major classes of mechanism are recognised: modulation of voltage-gated ion channels; enhancement of gamma-aminobutyric acid (GABA)-mediated inhibitory neurotransmission; and attenuation of glutamate-mediated excitatory neurotransmission. The principal pharmacological targets of currently available AEDs are highlighted in Table 1 and discussed further below. -
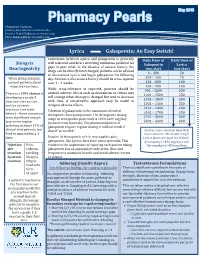
Lyrica Gabapentin: an Easy Switch!
Pharmacist Contacts: [email protected]; [email protected]; [email protected] Lyrica Gabapentin: An Easy Switch! Conversion between Lyrica and gabapentin is generally Daily Dose of Daily Dose of Shingrix well tolerated and direct switching minimizes potential for Gabapentin Lyrica Reactogenicity gaps in pain relief. In the absence of seizure history, the (mg/day) (mg/day) drugs can be directly interchanged; patients can be advised 0 – 300 50 to discontinue Lyrica and begin gabapentin the following When giving Shingrix, day. Patients with a seizure history should be cross-tapered 301 – 450 75 counsel patients about over 1 – 4 weeks. 451 – 600 100 expected reactions. 601 – 900 150 While cross-tolerance is expected, patients should be 901 – 1200 200 advised adverse effects such as drowsiness or edema may There is a 10% chance of 1201 – 1500 250 still emerge when therapy is changed but tend to decrease developing a grade 3 1501 – 1800 300 injection site reaction with time. A conservative approach may be useful to 1801 – 2100 350 and/or systemic mitigate adverse effects. reactions (see table 2101 – 2400 400 Titration of gabapentin to the maximum tolerated 2401 – 2700 450 below) – these symptoms therapeutic dose is important. The therapeutic dosing 2701 – 3000 500 were significant enough range in neuropathic pain trials is 1800-3600 mg/day to prevent regular (normal renal function). The pharmacokinetics of 3001 – 3600 600 activities in about 17% of gabapentin require regular dosing, it will not work if clinical trial patients, but dosed “as needed.” Studies show minimal benefit & tend to pass within 2-3 more adverse effects when high days. -

Comparing Adverse Effects of Analgesic Strategies For
Comparing Adverse Effects daily and weekly time during which higher than that of the only ATC opi- their pain interfered with their mood oids group and 17 times higher than of Analgesic Strategies for or activities, and they indicated the that of the only PRN opioids group. Chronic Cancer Pain amount of relief they received from Source: J Pain Symptom Manage. 2007;33(1):67–77. their pain medicine in the previ- Nausea, vomiting, drowsiness, lack ous week. They also completed the of energy, urinary retention—some- Karnofsky Performance Status (KPS), Clopidogrel: Best Results times the adverse effects of the anal- which measures patients’ ability to per- Pre- or Post-PCI? gesic medication are enough to derail form activities of daily living and their pain management for patients with need for caregiver assistance. When is the best time to give the anti- cancer. It would help to understand There were no significant differ- platelet clopidogrel to patients sched- the relationships between the type of ences between any of the four groups uled for diagnostic coronary angiogra- analgesic prescription and the preva- in terms of pain intensity or amount of phy—before ad hoc coronary stenting lence and severity of adverse effects. time spent in pain. Total pain interfer- or immediately after? Researchers from But not much information is available, ence scores, however, were significantly University of Debrecen, Debrecen, say researchers from the University higher in the ATC plus PRN opioids Hungary and Medical University of of California, San Francisco; the Uni- group than in the no opioids group. Vienna and Wilhelminenhospital, both versity of Nebraska, Omaha; and the The ATC plus PRN opioids group in Vienna, Austria say starting treat- University of Texas Southwestern Med- also had significantly lower functional ment before percutaneous coronary ical Center, Dallas. -

Membrane Stabilizer Medications in the Treatment of Chronic Neuropathic Pain: a Comprehensive Review
Current Pain and Headache Reports (2019) 23: 37 https://doi.org/10.1007/s11916-019-0774-0 OTHER PAIN (A KAYE AND N VADIVELU, SECTION EDITORS) Membrane Stabilizer Medications in the Treatment of Chronic Neuropathic Pain: a Comprehensive Review Omar Viswanath1,2,3 & Ivan Urits4 & Mark R. Jones4 & Jacqueline M. Peck5 & Justin Kochanski6 & Morgan Hasegawa6 & Best Anyama7 & Alan D. Kaye7 Published online: 1 May 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Purpose of Review Neuropathic pain is often debilitating, severely limiting the daily lives of patients who are affected. Typically, neuropathic pain is difficult to manage and, as a result, leads to progression into a chronic condition that is, in many instances, refractory to medical management. Recent Findings Gabapentinoids, belonging to the calcium channel blocking class of drugs, have shown good efficacy in the management of chronic pain and are thus commonly utilized as first-line therapy. Various sodium channel blocking drugs, belonging to the categories of anticonvulsants and local anesthetics, have demonstrated varying degrees of efficacy in the in the treatment of neurogenic pain. Summary Though there is limited medical literature as to efficacy of any one drug, individualized multimodal therapy can provide significant analgesia to patients with chronic neuropathic pain. Keywords Neuropathic pain . Chronic pain . Ion Channel blockers . Anticonvulsants . Membrane stabilizers Introduction Neuropathic pain, which is a result of nervous system injury or lives of patients who are affected. Frequently, it is difficult to dysfunction, is often debilitating, severely limiting the daily manage and as a result leads to the progression of a chronic condition that is, in many instances, refractory to medical This article is part of the Topical Collection on Other Pain management. -

Antidepressants the Old and the New
Antidepressants The Old and The New October, 1998 iii In 1958 researchers discovered that imipramine had antidepressant 1 activity. Since then, a number of antidepressants have been HIGHLIGHTS developed with a variety of pharmacological mechanisms and side effect profiles. All antidepressants show similar efficacy in the treatment of depression when used in adequate dosages. Choosing the most PHARMACOLOGY & CLASSIFICATION appropriate agent depends on specific patient variables, The mechanism of action for antidepressants is not entirely clear; concurrent diseases, concurrent drugs, and cost. however they are known to interfere with neurotransmitters. Non-TCA antidepressants such as the SSRIs have become Tricyclic antidepressants (TCAs) block the reuptake of both first line agents in the treatment of depression due to their norepinephrine (NE) and serotonin (5HT). The relative ratio of relative safety and tolerability. Each has its own advantages and their effect on NE versus 5HT varies. The potentiation of NE and disadvantages for consideration in individualizing therapy. 5HT results in changes in the neuroreceptors and is thought to be TCAs may be preferred in patients who do not respond to or the primary mechanism responsible for the antidepressant effect. tolerate other antidepressants, have chronic pain or migraine, or In addition to the effects on NE and 5HT, TCAs also block for whom drug cost is a significant factor. muscarinic, alpha1 adrenergic, and histaminic receptors. The extent of these effects vary with each agent resulting in differing Secondary amine TCAs (desipramine and nortriptyline) have side effect profiles. fewer side effects than tertiary amine TCAs. Maintenance therapy at full therapeutic dosages should be Selective serotonin-reuptake inhibitors (SSRIs) block the 2 considered for patients at high risk for recurrence. -

The American Journal Of
The American Journal of Psychiatry Residents’ Journal July 2015 Volume 10 Issue 7 Inside IN THIS ISSUE 2 New Formats and New Opportunities: The Time to Get Involved is “Now”! Rajiv Radhakrishnan, M.B.B.S., M.D. 3 Prevention of Posttraumatic Stress Disorder: Predicting Response to Trauma Jennifer H. Harris, M.D. 7 Weight Gain in Patients With Schizophrenia: A Recipe For Timely Intervention Ammar El Sara, M.B.Ch.B. 10 Hyperprolactinemia and Antipsychotics: Update for the Training Psychiatrist Stephanie Pope, M.D. 13 A Clinical Case Conference on Spiritual Growth and Healing Elizabeth S. Stevens, D.O. This issue of the Residents’ Journal features a variety of topics. Jennifer H. Har- ris, M.D., discusses prevention of posttraumatic stress disorder, with an overview 15 Priapism: A Rare but Serious of various responses to trauma. Ammar El Sara, M.B.Ch.B., presents a review of Side Effect of Trazodone clinically applicable evidence-based interventions targeting obesity in schizophre- Kamalika Roy, M.D. nia patients. Stephanie Pope, M.D., examines antipsychotic-induced hyperprolac- 17 Classifying Psychopathology: tinemia, including variables affecting prolactin and clinical implications. Elizabeth Mental Kinds and Natural Kinds S. Stevens, D.O., discusses several psychological, social, and spiritual developmen- Reviewed by Aaron J. Hauptman, tal frameworks in a clinical case conference. Kamalika Roy, M.D., presents a case M.D. of priapism as a side effect of trazodone in a middle-aged patient. Lastly, Aaron J. Hauptman, M.D., offers his review of the book Classifying Psychopathology: Mental 18 Residents’ Resources Kinds and Natural Kinds. Editor-in-Chief Associate Editors Editors Emeriti Rajiv Radhakrishnan, M.B.B.S., M.D. -

Adverse Effects of First-Line Pharmacologic Treatments of Major Depression in Older Adults
Draft Comparative Effectiveness Review Number xx Adverse Effects of First-line Pharmacologic Treatments of Major Depression in Older Adults Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 5600 Fishers Lane Rockville, MD 20857 www.ahrq.gov This information is distributed solely for the purposes of predissemination peer review. It has not been formally disseminated by the Agency for Healthcare Research and Quality. The findings are subject to change based on the literature identified in the interim and peer-review/public comments and should not be referenced as definitive. It does not represent and should not be construed to represent an Agency for Healthcare Research and Quality or Department of Health and Human Services (AHRQ) determination or policy. Contract No. 290-2015-00012I Prepared by: Will be included in the final report Investigators: Will be included in the final report AHRQ Publication No. xx-EHCxxx <Month, Year> ii Purpose of the Review To assess adverse events of first-line antidepressants in the treatment of major depressive disorder in adults 65 years or older. Key Messages • Acute treatment (<12 weeks) with o Serotonin norepinephrine reuptake inhibitors (SNRIs) (duloxetine, venlafaxine), but not selective serotonin reuptake inhibitors (SSRIs) (escitalopram, fluoxetine) led to a greater number of adverse events compared with placebo. o SSRIs (citalopram, escitalopram and fluoxetine) and SNRIs (duloxetine and venlafaxine) led to a greater number of patients withdrawing from studies due to adverse events compared with placebo o Details of the contributing adverse events in RCTs were rarely reported to more clearly characterize what adverse events to expect. -

Insomnia Disorder a VA Clinician’S Guide to Managing Insomnia Disorder (2019) Contents Insomnia Disorder
Insomnia Disorder A VA Clinician’s Guide to Managing Insomnia Disorder (2019) Contents Insomnia Disorder .................................................................................................... 3 Risks in elderly patients and patients with dementia .................................15 Background ................................................................................................................ 3 Provider perceptions vs reality ............................................................................16 Figure 1. Stepped Care for Management of Insomnia Disorder .............. 3 Figure 5. Weighing the potential risks versus benefits of Table 1. Brief summary of the ISI ......................................................................... 4 medication use .........................................................................................................16 Figure 2. Acute Insomnia to Insomnia Disorder ............................................ 5 Doxepin ........................................................................................................................17 Clinical Pearl ............................................................................................................... 5 Figure 6. Doxepin Use .............................................................................................18 Figure 3. Common causes of sleep disturbance ........................................... 6 Ramelteon ...................................................................................................................18 -

Doxepin (Dox-E-Pin) Description: Tricyclic Antidepressant; Antihistamine Other Names for This Medication: Sinequan®, Silenor® Common Dosage Forms: Veterinary: None
Prescription Label Patient Name: Species: Drug Name & Strength: Directions (amount to give how often & for how long): Prescribing Veterinarian's Name & Contact Information: Refills: [Content to be provided by prescribing veterinarian] Doxepin (dox-e-pin) Description: Tricyclic Antidepressant; Antihistamine Other Names for this Medication: Sinequan®, Silenor® Common Dosage Forms: Veterinary: None. Human: 3 mg, 6 mg, 10 mg, 25 mg, 50 mg, 75 mg, 100 mg, & 150 mg capsules; 10 mg/mL oral liquid concentrates. This information sheet does not contain all available information for this medication. It is to help answer commonly asked questions and help you give the medication safely and effectively to your animal. If you have other questions or need more information about this medication, contact your veterinarian or pharmacist. Key Information When used as an antihistamine, doxepin should be used on a regular, ongoing basis in animals that respond to it. This drug works better if used before exposure to an allergen (eg, pollens). When used for behavior modification, it may take several days to weeks to determine if doxepin is effective. May be given with or without food. If your animal vomits or acts sick after receiving the drug on an empty stomach, try giving the next dose with food or a small treat. If vomiting continues, contact your veterinarian. Most common side effects are sleepiness, dry mouth, and constipation. Be sure your animal always has access to plenty of fresh, clean water. Rare side effects that can be serious (contact veterinarian immediately) include abnormal bleeding, lack of an appetite, seizures, collapse, or profound sleepiness.