Population Differentiation in the European Roe Deer by Non-Metric Skull Traits in Germany
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Health-Among-The-Elderly-In-Germany.Pdf
BEITRÄGE ZUR BEVÖLKERUNGSWISSENSCHAFT - SERIES ON POPULATION STUDIES Book series published by the German Federal Institute for Population Research (Bundesinstitut für Bevölkerungsforschung) Volume 46 Gabriele Doblhammer (Ed.) Health Among the Elderly in Germany New Evidence on Disease, Disability and Care Need Barbara Budrich Publishers Opladen • Berlin • Toronto 2015 All rights reserved. No part of this publication may be reproduced, stored in or introduced into a retrieval system, or transmitted, in any form, or by any means (electronic, mechanical, photocopying, recording or otherwise) without the prior written permission of Barbara Budrich Publishers. Any person who does any unauthorized act in relation to this publication may be liable to criminal prosecution and civil claims for damages. You must not circulate this book in any other binding or cover and you must impose this same condition on any acquirer. A CIP catalogue record for this book is available from Die Deutsche Bibliothek (The German Library) © 2015 by Barbara Budrich Publishers, Opladen, Berlin & Toronto www.barbara-budrich.net ISBN 978-3-8474-0606-8 eISBN 978-3-8474-0288-6 Das Werk einschließlich aller seiner Teile ist urheberrechtlich geschützt. Jede Verwertung außerhalb der engen Grenzen des Urheberrechtsgesetzes ist ohne Zustimmung des Verlages unzulässig und strafbar. Das gilt insbesondere für Vervielfältigungen, Übersetzungen, Mikroverfilmungen und die Einspeicherung und Verarbeitung in elektronischen Systemen. Die Deutsche Bibliothek – CIP-Einheitsaufnahme Ein Titeldatensatz für die Publikation ist bei der Deutschen Bibliothek erhältlich. Verlag Barbara Budrich Barbara Budrich Publishers Stauffenbergstr. 7. D-51379 Leverkusen Opladen, Germany 86 Delma Drive. Toronto, ON M8W 4P6 Canada www.barbara-budrich.net Picture credits/Titelbildnachweis: Microstockfish, Artalis - Fotolia.com and Dr. -

Ancestors of Charles Rietesel
Ancestors of Charles Rietesel Generation 1 1. Charles Rietesel, son of Joachim Carl Friedrich Rietesel and Amalia Johanna Voss, was born on October 14, 1877 in Stralsund, Mecklenburg-Vorpommern, Germany. He died on January 27, 1949 in McHenry County, IL. He married Karolina Tron on October 26, 1904 in Vanderburgh, Indiana. She was born on September 11, 1875 in Posey County, IN. She died on July 28, 1964 in Cook County, IL. Notes for Charles Rietesel: In the 1910 Census he is listed as being born in Illinois but a WWII draft card explicitly names Stralsund as his birthplace and uses the 'Rietesel' spelling. In 1930 they are living in McHenry Illinois (Census spelling=Rietzel). They own a home worth $5,000 and he is a mechanic. Notes for Karolina Tron: Known as Caroline. Generation 2 2. Joachim Carl Friedrich Rietesel, son of Johann Joachim Riedesel and Maria Dorothea Holfreter, was born on February 21, 1851 in Muucks, Nordvorpommern, Mecklenburg- Vorpommern, Germany. He died between 1910-1920 in probably Chicago. He married Amalia Johanna Voss on February 20, 1876 in Stralsund, Mecklenburg-Vorpommern, Germany. 3. Amalia Johanna Voss, daughter of Johann Friedrich Voß and Johanne Marie Brodthagen, was born on May 15, 1854 in Gutglück, Nordvorpommern, Mecklenburg-Vorpommern, Germany. She died after 1940. Notes for Joachim Carl Friedrich Rietesel: Almost certainly the party that arrived in Baltimore on the ship Leipzig out of Bremen on July 7, 1880 consisted of Friedrich, his wife (whose name is obliterated) and Carl age 2 1/2 years. Friedrich's age and that of Carl/Charles perfectly matches a "Rietzel" household in Chicago in 1900. -

LKVR EBAW Tourenplan 1 Schadstoffsammlung 2020
Tourenplan für die 1. Schadstoffsammlung 2020 im Landkreis Vorpommern-Rügen Entsorgungsgebiet: Nordvorpommern Zeitraum: 10. Februar - 6. März 2020 Schadstoffe können in haushaltsüblichen Mengen – max. 20 Liter/kg je Abfallart am Schadstoffmobil abgegeben werden. Autopflegemittel Farben, Lacke, Lösungsmittel, Klebstoffe, Desinfektionsmittel Holzschutz-, Pflanzenschutz- und Schädlingsbekämpfungsmittel Säuren, Laugen, Haushaltsreiniger, Kosmetika, Haushaltschemikalien Leuchtstofflampen, Quecksilberdampflampen, Thermometer, Energiesparlampen Gifte, Chemikalien Ölverunreinigte Abfälle (Putzlappen u. ä.) Spraydosen mit schädlichen Resten (z. B. Spray zur Reinigung von Backöfen) Weiterhin werden elektrische Haushaltskleingeräte bis zur Länge, Breite und Tiefe von jeweils maximal 30 cm mitgenommen, wie z. B. Bügeleisen, Toaster, Mobiltelefone. Diese Schadstoffe dürfen nicht unbeaufsichtigt an den Stellplätzen des Schadstoffmobiles abgestellt werden. Verkaufsverpackungen wie restentleerte Dosen und Eimer werden nicht mitgenommen. Diese entsorgen Sie bitte über den Gelben Sack / Gelbe Tonne. 1. Woche: 10. Februar - 15. Februar 2020 Amt Ort Stellplatz Datum Uhrzeit Altenpleen Neuenpleen Am IGLU 10.02.2020 Montag 09:00-09:15 Altenpleen Altenpleen Stralsunder Str. 27 / P Schule 10.02.2020 Montag 09:30-09:45 Altenpleen Groß Mohrdorf Feuerwehr 10.02.2020 Montag 10:00-10:15 Altenpleen Hohendorf Buswendeplatz 10.02.2020 Montag 10:30-10:45 Altenpleen Klausdorf Am IGLU 10.02.2020 Montag 11:00-11:15 Altenpleen Barhöft Hafen 10.02.2020 Montag 11:30-11:45 Altenpleen Prohn Ringstr., Höhe Schule 10.02.2020 Montag 12:30-12:45 Altenpleen Prohn P - Edeka Markt 10.02.2020 Montag 13:00-13:30 Altenpleen Krönnevitz Gutshaus 10.02.2020 Montag 13:45-14:00 Altenpleen Schmedshagen Dorfplatz Ringstr. / Am IGLU 10.02.2020 Montag 14:15-14:45 Altenpleen Groß Kedingshagen Kedingshäger Straße 10.02.2020 Montag 15:00-15:15 Altenpleen Klein Kedingshagen P - Kastanienallee 10.02.2020 Montag 15:30-15:45 Barth Hermannshg. -

Jahresbericht 2018
DRK Kreisverband Nordvorpommern e.V. Jahresbericht 2018 (Tätigkeitsnachweis) Inhaltsverzeichnis I. Das Deutsche Rote Kreuz – der Kreisverband Nordvorpommern e.V. S. 1-3 II. Unsere Strukturen der Vereinsarbeit S. 3 III. Ehrenamtliche Arbeit der Ortsvereine S. 3-5 IV. Bereitschaften - Verpflegungsgruppe und MTF S. 6 V. Gemeinschaften - Wasserwacht und Jugendrotkreuz S. 7 VI. Erste Hilfe Ausbildungen S. 8 VII. Besonderer Höhepunkt S. 9 VIII. 11. EhrenamtMesse S. 10 DRK Kreisverband Nordvorpommern e.V. • Körkwitzer Weg 43 • 18311 Ribnitz-Damgarten DRK Kreisverband Nordvorpommern e.V. I. Das Deutsche Rote Kreuz – der Kreisverband Nordvorpommern e.V. Die Geschichte des Deutschen Roten Der hilfebedürftige Mensch Kreuzes ist mehr als 150 Jahre alt. So Wir schützen und helfen dort, wo wurde 1863 in Baden-Württemberg die menschliches Leiden zu verhüten und zu erste Rotkreuzgesellschaft der Welt lindern ist. gegründet. Die Idee, Menschen allein nach dem Maß der Not zu helfen, ohne auf Hautfarbe, Religion oder Nationalität zu Die unparteiliche Hilfeleistung achten, geht auf den Schweizer Henry Alle Hilfebedürftigen haben den gleichen Dunant zurück. Anspruch auf Hilfe, ohne Ansehen der Nationalität, der Rasse, der Religion, des Geschlechts, der sozialen Stellung oder der politischen Überzeugung. Wir setzen die verfügbaren Mittel allein nach dem Maß der Not und der Dringlichkeit der Hilfe ein. Unsere freiwillige Hilfeleistung soll die Selbsthilfekräfte der Hilfebedürftigen wiederherstellen. Neutral im Zeichen der Menschlichkeit Wir sehen uns ausschließlich als Helfer und Anwälte der Hilfebedürftigen und enthalten uns zu jeder Zeit der Teilnahme an politischen, rassischen oder religiösen Auseinandersetzungen. Wir sind jedoch nicht bereit, Unmenschlichkeit hinzunehmen und erheben deshalb, wo geboten, unsere Stimme gegen ihre Ursachen. -

1986L0465 — Nl — 13.03.1997 — 003.001 — 1
1986L0465 — NL — 13.03.1997 — 003.001 — 1 Dit document vormt slechts een documentatiehulpmiddel en verschijnt buiten de verantwoordelijkheid van de instellingen ►B RICHTLIJN VAN DE RAAD van 14 juli 1986 betreffende de communautaire lijst van agrarische probleemgebieden in de zin van Richtlijn 75/ 268/EEG (Duitsland) (86/465/EEG) (PB L 273 van 24.9.1986, blz. 1) Gewijzigd bij: Publicatieblad nr. blz. datum ►M1 Richtlijn 89/586/EEG van de Raad van 23 oktober 1989 L 330 1 15.11.1989 ►M2 Beschikking 91/26/EEG van de Commissie van 18 december 1990 L 16 27 22.1.1991 ►M3 Richtlijn 92/92/EEG van de Raad van 9 november 1992 L 338 1 23.11.1992 ►M4 gewijzigd bij Beschikking 93/226/EEG van de Commissie van 22 april L 99 1 26.4.1993 1993 ►M5 gewijzigd bij Beschikking 97/172/EG van de Commissie van 10 L 72 1 13.3.1997 februari 1997 ►M6 gewijzigd bij Beschikking 95/6/EG van de Commissie van 13 januari L 11 26 17.1.1995 1995 1986L0465 — NL — 13.03.1997 — 003.001 — 2 ▼B RICHTLIJN VAN DE RAAD van 14 juli 1986 betreffende de communautaire lijst van agrarische probleemge- bieden in de zin van Richtlijn 75/268/EEG (Duitsland) (86/465/EEG) DE RAAD VAN DE EUROPESE GEMEENSCHAPPEN, Gelet op het Verdrag tot oprichting van de Europese Economische Gemeenschap, Gelet opRichtlijn 75/268/EEG van de Raad van 28 april1975 betref- fende de landbouw in bergstreken en in sommige probleemgebieden (1), laatstelijk gewijzigd bij Verordening (EEG) nr. -

Die Spätpleistozäne Bis Frühholozäne Beckenentwicklung in Mecklenburg-Vorpommern - Untersuchungen Zur Stratigraphie, Geomorphologie Und Geoar- Chäologie
GREIFSWALDER GEOGRAPHISCHE ARBEITEN ___________________________________________________________________________ Geographisches Institut der Ernst-Moritz-Arndt-Universität Greifswald Band 24 Die spätpleistozäne bis frühholozäne Beckenentwicklung in Mecklenburg-Vorpommern - Untersuchungen zur Stratigraphie, Geomorphologie und Geoar- chäologie von Knut Kaiser GREIFSWALD 2001 _______________________________________________________________________ ERNST-MORITZ-ARNDT-UNIVERSITÄT GREIFSWALD Impressum ISBN: 3-86006-183-6 Ernst-Moritz-Arndt-Universität Greifswald Herausgabe: Konrad Billwitz Redaktion: Knut Kaiser Layout: Knut Kaiser Grafik: Petra Wiese, Knut Kaiser Herstellung: Vervielfältigungsstelle der Ernst-Moritz-Arndt-Universität, KIEBU-Druck Greifswald Kontakt: Dr. Knut Kaiser, Ernst-Moritz-Arndt-Universität, Geographisches Institut, Jahnstraße 16, D-17487 Greifswald, e-mail: [email protected] ________________________________________________________________________________________ Für den Inhalt ist der Autor verantwortlich. Inhaltsverzeichnis Vorwort 5 1. Einführung 7 1.1 Allgemeines 7 1.2 Spezielle Fragestellungen 8 2. Methodik 11 2.1 Geländearbeiten 11 2.2 Laborarbeiten 12 3. Paläoklimatische, stratigraphische und paläogeographische Grundlagen 15 3.1 Spätpleistozäne bis frühholozäne Klimaentwicklung im nördlichen Mitteleuropa 15 3.2 Regionale Stratigraphie und Paläogeographie 16 3.2.1 Stratigraphie 16 3.2.2 Paläogeographie 18 3.3 Regionale Radiokohlenstoffdaten 20 3.3.1 Allgemeines 20 3.3.2 Datenvorlage 22 3.3.3 Auswertung 24 -

AMTSBLATT Der Hansestadt Stralsund
AMTSBLATT der Hansestadt Stralsund Herausgeber: Hansestadt Stralsund • Der Oberbürgermeister Nr. 8 12. Jahrgang Stralsund, 27.07.2002 Inhalt Seite Haushaltssatzung und Haushaltsplan 2 der Hansestadt Stralsund für das Haushaltsjahr 2002 Frühzeitige Bürgeranhörung 2 Bebauungsplan Nr. 38 der Hansestadt Stralsund „Hafen und Uferbereich an der Schwedenschanze“ Frühzeitige Bürgeranhörung 2 Bebauungsplan Nr. 50 der Hansestadt Stralsund „Technologiepark Prohner Straße“ Öffentliche Bekanntmachung 3 des Bebauungsplanes Nr. 36 der Hansestadt Stralsund „Stadtgebiet Grünhufe westlich der Dorfstraße“ Öffentliche Auslegung 3 Bebauungsplan Nr. 44 der Hansestadt Stralsund „Kleiner Wiesenweg - südlicher Teil“ Bekanntmachung der Mitglieder 3 des Kreiswahlausschusses für die Wahl zum 4. Landtag in Mecklenburg-Vorpommern für die Wahlkreise 23-Nordvorpommern I, 24-Nordvorpommern II und 25-Nordvorpommern III/Stralsund I Sitzung des Kreiswahlausschusses 4 der Wahlkreise 23, 24 und 25 über die Zulassung der Kreiswahlvorschläge für die Landtagswahl am 22. September 2002 Ausschreibung 4 Vergabe der Jugendarbeit in der Jugendfreizeiteinrichtung Wiesenstraße 9, 18437 Stralsund Programm der Volkshochschule Stralsund 4 Monat August Impressum 4 Amtsblatt der Hansestadt Stralsund - Nr. 8 Amtliche Bekanntmachung nicht genehmigt.Es bleibt bei der genehmigungsfreien Höchst- 1. Haushaltssatzung und Haushaltsplan der Han- grenze in Höhe von 10 v.H. der Erträge des Erfolgsplanes (58.000 EUR). sestadt Stralsund für das Haushaltsjahr 2002 Mit dieser Bekanntmachungsanordnung wird nach § 5 Abs.4 Satz 1 Auf Grund der §§ 47 ff KV M-V wird nach Beschluss der Bürger- KV M-V die Haushaltssatzung 2002 öffentlich bekannt gemacht. schaft vom 07.03.2002 – und mit Genehmigung der Rechtsauf- sichtsbehörde – folgende Haushaltssatzung erlassen: Die Haushaltssatzung und der Haushaltsplan 2002 sowie dessen Anlagen liegen zur Einsichtnahme in der Bürgerinformation der Han- §1 sestadt Stralsund, Alter Markt 9, sowie im Kämmereiamt, Heilgeiststr. -

CSC Report Laura Roth Geändert 07.04
CLIMATE SERVICE CENTER CSC Report 19 Decision Support Tools as Instruments to facilitate Climate Change Adaptation The case of the BalticClimate Toolkit for adaptation in the German Baltic Sea region Cover: © 2011 BalticClimate/toolkit.balticclimate.org Citation: L. Roth, R. Birner, C. Henriksen & M. Schaller (2014): Decision Support Tools as Instruments to facilitate Climate Change Adaption – The Case of the BalticClimate Toolkit for adaptation in the German Baltic Sea region, CSC Report 19, Climate Service Center, Germany Decision Support Tools as Instruments to facilitate Climate Change Adaptation The case of the BalticClimate Toolkit for adaptation in the German Baltic Sea region Internship-Report by Laura Roth Based on the Master of Science Thesis February 2014 Host Organization: Climate Service Center (CSC), Germany Sending Organizations: University of Hohenheim (UHOH), Germany University of Copenhagen (KU), Denmark Supervisor at CSC: Dr. Michaela Schaller, Department Head “Management of Natural Resources” Supervisors from Sending Organizations: Prof. Dr. Regina Birner (UHOH), Department Head “Social and Institutional Change in Agricultural Development” Prof. Christian Bugge Henriksen (KU), Associated Professor “Climate Change and Crop Ecology” Content Content....................................................................................................................................1 Acronyms................................................................................................................................2 List -

Bahnhof - Von Grimmen Nach Tribsees Fortzusetzen
HIN & WEG Pilgern durch Feld und Wald 5 Route: Grimmen - Vietlipp – „Alter Bahndamm“ – Strelow – Turow – Voigtsdorf – Zarnekow – „Alter Bahndamm“ – Stadtwald – Tribsees Orientierung: größtenteils amtliche Radwegweiser Länge, Dauer: ca. 29 km (plus Rückweg) Tipp: Gut geeignet als Rundtour per Rad mit Rückweg entlang der L 19. Alternativ morgens erst per (Schul-)Bus von Tribsees oder Grimmen zum entfernten Startpunkt einer Fußwanderung zurück. Die Bahn kommt – aber zum Glück nicht mehr hier entlang, denn hier können Sie ganz entspannt und in aller Ruhe die Landschaft genießen. Der Alte Bahndamm gehört jetzt den Radlern und Wanderern, Sie müssen sich ihn höchstens mit Haselnüssen, Eicheln, Holunderbeeren oder aber auch mit Schwalben, Neuntöter und Spatzen teilen. Vielleicht begeben Sie sich unterwegs ja auch auf die Suche nach dem Schlossgespenst in der Wasserburg Turow - ein Picknick im Park ist alle Natur-Erlebnisse auf Wanderwegen mal empfehlenswert, bevor Sie sich wieder in Richtung Ziel Ihrer Reise auf den Weg machen. Nicht von ungefähr hat sich dereinst das Ackerbürgerstädtchen Tribsees hier angesiedelt, wo sich der Arm des Flüsschens Trebel schützend um die uralten Stadtmauern legt, um hier seine stetige Westpassage abrupt nach Südosten Impressum: bis zur Peene, dem Amazonas des Nordens, Text: NABU Nordvorpommern/R. Schmidt Bahnhof - von Grimmen nach Tribsees fortzusetzen. Layout: STADT LAND FLUSS Fotos: NABU Nordvorpommern/R. Schmidt © Geobasisdaten (Karten): Landesamt für innere Verwaltung Mecklenburg-Vorpommern (LAiV-MV) Gefördert durch die Gemeinschaftsinitiative Leader+, das Land M-V und Landkreis Nordvorpommern In Grimmen gelangen wir aus der Altstadt durch das Mühlentor Als nächstes erreichen wir Voigtsdorf. Wie viele Dörfer in dieser Vorbei an einer Schutzhütte, auf deren Höhe gegenüber ein und überqueren an der Poggendorfer Trebel die Friedrichstraße. -

Gemeindeverzeichnis Mecklenburg-Vorpommern 11.02.2020 Gebietsstand : 31.12.2018
Kassenärztliche Vereinigung Seite : 1 Mecklenburg-Vorpommern Gemeindeverzeichnis Mecklenburg-Vorpommern 11.02.2020 Gebietsstand : 31.12.2018 Amtlicher PLZ Gemeinde- Gemeinde Mittelbereich Kreis / Kreisregion Raumordnungsregion schlüssel 17033 13 071 107 Neubrandenburg, Stadt Neubrandenburg Neubrandenburg / Mecklenburg-Strelitz Mecklenburgische Seenplatte 17034 13 071 107 Neubrandenburg, Stadt Neubrandenburg Neubrandenburg / Mecklenburg-Strelitz Mecklenburgische Seenplatte 17036 13 071 107 Neubrandenburg, Stadt Neubrandenburg Neubrandenburg / Mecklenburg-Strelitz Mecklenburgische Seenplatte 17039 13 071 009 Beseritz Neubrandenburg-Umland Neubrandenburg / Mecklenburg-Strelitz Mecklenburgische Seenplatte 17039 13 071 010 Blankenhof Neubrandenburg-Umland Neubrandenburg / Mecklenburg-Strelitz Mecklenburgische Seenplatte 17039 13 071 019 Brunn Neubrandenburg-Umland Neubrandenburg / Mecklenburg-Strelitz Mecklenburgische Seenplatte 17039 13 071 104 Neddemin Neubrandenburg-Umland Neubrandenburg / Mecklenburg-Strelitz Mecklenburgische Seenplatte 17039 13 071 108 Neuenkirchen Neubrandenburg-Umland Neubrandenburg / Mecklenburg-Strelitz Mecklenburgische Seenplatte 17039 13 071 111 Neverin Neubrandenburg-Umland Neubrandenburg / Mecklenburg-Strelitz Mecklenburgische Seenplatte 17039 13 071 140 Sponholz Neubrandenburg-Umland Neubrandenburg / Mecklenburg-Strelitz Mecklenburgische Seenplatte 17039 13 071 141 Staven Neubrandenburg-Umland Neubrandenburg / Mecklenburg-Strelitz Mecklenburgische Seenplatte 17039 13 071 145 Trollenhagen Neubrandenburg-Umland -

Libellenbeobachtungen in Nordvorpommern Und Angrenzenden Gebieten
Libellula 18 (1/2): 49-53 1999 Libellenbeobachtungen in Nordvorpommern und angrenzenden Gebieten Kay Fuhrmann eingegangen: 3. März 1998 Summary Dragonfly observations in the north of Vorpommern and adjäncent regions - From 1994 to 1996, 39 dragonfly species were recorded at 28 different localities in the northeastern part of Mecklenburg-Vorpommern, Germany. Emphasize is given to the northernmost occurrence of Erythromma viridulum in Europe, Anax imperator and Svmpecma fnsca on Baltic islands, as well as a locality with a large population of Leurorrhinia albifrons. Zusammenfassung Von 1994 bis 1996 wurden im nördlichen Bereich Vorpommerns (Meck lenburg-Vorpommern) und einigen Gebieten des weiteren Umkreises insge samt 39 Libellenarten an 28 Fundorten festgestellt. Besonders hervorzuheben ist der nördlichste europäische Nachweis von Erythromma viridulum, die Inselvorkommen von Anax imperator und Sympecma fusca und ein großes Vorkommen von Leucorrhinia albifrons. Einleitung Der odonatologische Durchforschungsgrad Vorpommerns ist im Ver gleich zu anderen Bundesländern noch gering. Nur wenige Berichte in der Literatur werfen ein Licht auf das Vorkommen von Libellen dieses Raumes (z.B. Königstedt & Schmidt 1981. Mauersberger & Wagner 1990. Mauersberger 1989. Zessin 1986). In den Jahren 1994 - 1996 hatte ich die Gelegenheit. Libellen in Nordvorpommem und benachbarten Bereichen zu beobachten. Der folgende Bericht soll ein bescheidener Beitrag zur Kenntnis der Libellenfauna des genannten Gebietes sein. Kay Fuhrmann, Elritzenweg 23a, D-26127 Oldenburg E-Mail: kav.fuhrmamüSt-online.de 50 Kav Fuhrmann Methode und Untersuchungsgebiet Im Zeitraum 1994 - 1996 wurden zwischen Mitte Mai und Anfang Ok tober sporadisch verschiedene Gewässer z.T. mehrmalig angefahren. Ein deutlicher Schwerpunkt lag dabei in der Beobachtung an stehenden Ge wässern. Libellen wurden gekeschert, bestimmt und am Fangplatz wieder freigelassen. -
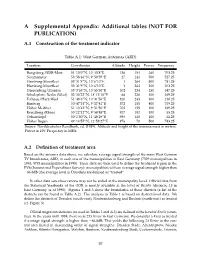
A Supplemental Appendix: Additional Tables (NOT for PUBLICATION)
A Supplemental Appendix: Additional tables (NOT FOR PUBLICATION) A.1 Construction of the treatment indicator Table A.1: West German Antennas (ARD) Location Coordinates Altitude Height Power Frequency Bungsberg, NDR-Mast 54◦13’0”N, 10◦43’8”E 136 154 260 703.25 Neumunster¨ 53◦58’46”N, 9◦50’59”E 27 141 500 527.25 Hamburg-Moorfleet 53◦31’9”N, 10◦6’10”E 1 264 500 751.25 Hamburg-Moorfleet 53◦31’9”N, 10◦6’10”E 1 264 100 203.25 Dannenberg/Zernien 53◦3’56”N, 10◦53’50”E 102 234 250 647.25 Scholzplatz, Berlin (West) 52◦30’22”N, 13◦13’10”E 66 220 100 189.25 Tofhaus/Harz-West 51◦48’6”N, 10◦31’56”E 820 243 100 210.25 Rimberg 50◦47’51”N, 9◦27’41”E 572 155 400 759.25 Hoher Meißner 51◦13’42”N, 9◦51’50”E 705 155 100 189.25 Kreuzberg (Rhon)¨ 50◦22’12”N, 9◦58’48”E 927 182 100 55.25 Ochsenkopf 50◦1’50”N, 11◦48’29”E 990 160 100 62.25 Hoher Bogen 49◦14’57”N, 12◦53’27”E 976 70 500 743.25 Source: Norddeutscher Rundfunk, ed. (1989). Altitude and height of the antenna mast in meters. Power in kW. Frequency in MHz. A.2 Definition of treatment area Based on the antenna data above, we calculate average signal strength of the main West German TV broadcaster, ARD, in each one of the municipalities in East Germany (7529 municipalities in 1993, 5793 municipalities in 1998).