Malus Sieversii: the Origin, Flavonoid Synthesis Mechanism, And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Use of Plant Growth Regulators for Branching of Nursery Trees in NY State Mario Miranda Sazo1 and Terence L
The Use of Plant Growth Regulators for Branching of Nursery Trees in NY State Mario Miranda Sazo1 and Terence L. Robinson2 1Cornell Cooperative Extension, Lake Ontario Fruit Program, Newark, NY 2Department of Horticulture, Cornell University, Geneva, NY he quality of nursery trees has a large impact on early pro- the use of Maxcel has been adopted by Italian nurserymen and is a duction and profitability of high density systems. Today, key step for the successful production of well feathered “knip-boom” nurserymen are not only asked to produce trees of good cali- apple trees. Depending on cultivar, Italian nurserymen apply from 1 T per but also highly to 4 Maxcel treatments with spray intervals of 5-7 days depending A new plant growth regulator, cyclanilide branched trees for on temperatures after application (Dr. Walter Guerra, Italy, personal “ the Tall Spindle communication). Since 2009, a new branching agent, cyclanilide (Tradename=Tiberon) very effectively system with short, (Tiberon formulated by Bayer Environmental Science, N.C. USA), induced lateral branching in nursery well positioned lat- has been available in the US for use in outdoor nurseries of apple, trees of 4 apple cultivars in the warm eral branches with sweet cherry, pear, and plum in Florida, Idaho, Oregon, Michigan, and humid year of 2010 in NY State. wide crotch angles. and Washington States. It is not currently registered for tree fruit This has required nursery use in New York State, Europe, or elsewhere. However, it also stopped shoot growth nurseries to im- Studies of the effects of Tiberon for branch induction of nursery for several weeks and reduced final prove their man- apple trees (and other fruit species) were conducted by Dr. -

Apples Catalogue 2019
ADAMS PEARMAIN Herefordshire, England 1862 Oct 15 Nov Mar 14 Adams Pearmain is a an old-fashioned late dessert apple, one of the most popular varieties in Victorian England. It has an attractive 'pearmain' shape. This is a fairly dry apple - which is perhaps not regarded as a desirable attribute today. In spite of this it is actually a very enjoyable apple, with a rich aromatic flavour which in apple terms is usually described as Although it had 'shelf appeal' for the Victorian housewife, its autumnal colouring is probably too subdued to compete with the bright young things of the modern supermarket shelves. Perhaps this is part of its appeal; it recalls a bygone era where subtlety of flavour was appreciated - a lovely apple to savour in front of an open fire on a cold winter's day. Tree hardy. Does will in all soils, even clay. AERLIE RED FLESH (Hidden Rose, Mountain Rose) California 1930’s 19 20 20 Cook Oct 20 15 An amazing red fleshed apple, discovered in Aerlie, Oregon, which may be the best of all red fleshed varieties and indeed would be an outstandingly delicious apple no matter what color the flesh is. A choice seedling, Aerlie Red Flesh has a beautiful yellow skin with pale whitish dots, but it is inside that it excels. Deep rose red flesh, juicy, crisp, hard, sugary and richly flavored, ripening late (October) and keeping throughout the winter. The late Conrad Gemmer, an astute observer of apples with 500 varieties in his collection, rated Hidden Rose an outstanding variety of top quality. -

APPLE (Fruit Varieties)
E TG/14/9 ORIGINAL: English DATE: 2005-04-06 INTERNATIONAL UNION FOR THE PROTECTION OF NEW VARIETIES OF PLANTS GENEVA * APPLE (Fruit Varieties) UPOV Code: MALUS_DOM (Malus domestica Borkh.) GUIDELINES FOR THE CONDUCT OF TESTS FOR DISTINCTNESS, UNIFORMITY AND STABILITY Alternative Names:* Botanical name English French German Spanish Malus domestica Apple Pommier Apfel Manzano Borkh. The purpose of these guidelines (“Test Guidelines”) is to elaborate the principles contained in the General Introduction (document TG/1/3), and its associated TGP documents, into detailed practical guidance for the harmonized examination of distinctness, uniformity and stability (DUS) and, in particular, to identify appropriate characteristics for the examination of DUS and production of harmonized variety descriptions. ASSOCIATED DOCUMENTS These Test Guidelines should be read in conjunction with the General Introduction and its associated TGP documents. Other associated UPOV documents: TG/163/3 Apple Rootstocks TG/192/1 Ornamental Apple * These names were correct at the time of the introduction of these Test Guidelines but may be revised or updated. [Readers are advised to consult the UPOV Code, which can be found on the UPOV Website (www.upov.int), for the latest information.] i:\orgupov\shared\tg\applefru\tg 14 9 e.doc TG/14/9 Apple, 2005-04-06 - 2 - TABLE OF CONTENTS PAGE 1. SUBJECT OF THESE TEST GUIDELINES..................................................................................................3 2. MATERIAL REQUIRED ...............................................................................................................................3 -

Apple Breeding: a Study of Jonathan Crosses
J une, 1928 Research Bulletin No. 116 A.pple Breeding: A Study of Jonathan Crosses By H. L . LANTZ AGRICULTURAL EXPERIMENT STATION IOWA STATE COLLEGE OF AGRICULTURE AND MECHANIC ARTS C. F. CURTISS, Director POMOLOGY SECTION AMES, IOWA SUMMARY Two hundred seventy-three Jonathan seedlings produced by 11 crosses form the basis of the records in this bulletin. These studies show clearly that the progenies produced by the different crosses of Jonathan differ or vary in many important char acteristics. These variations are attributed to differences in the genetic constitution of the Eeveral varieties which were used in combination with Jonathan, and indicate the relative value of these varieties as parents when crossed with Jonathan. The range of variation in the seedlings of these progenies is gen erally quite wide both as to tree and fruit. Differences in the horti cultural characteristics such as occur in size, form, color, flavor, quality and season of the seedling fruits suggest that multiple fac tors are concerned, which produce the many variations observed. Jonathan appears to carry as partially dominant factors for medi um and below medium size of fruit, but it evidently carries factors also for large and for small size. Factors for roundish, conic, ob late and oblong fruit forms are present. Jonathan appears to be homozygous for red skin color but is heterozygous for pattern and carries factors for both fine and coarse grained flesh, for juiciness, for both acid and sweet flavor and for very good as well as for poor quality. The factors which control the season of the fruit are evi dently complex. -

Variety Description Origin Approximate Ripening Uses
Approximate Variety Description Origin Ripening Uses Yellow Transparent Tart, crisp Imported from Russia by USDA in 1870s Early July All-purpose Lodi Tart, somewhat firm New York, Early 1900s. Montgomery x Transparent. Early July Baking, sauce Pristine Sweet-tart PRI (Purdue Rutgers Illinois) release, 1994. Mid-late July All-purpose Dandee Red Sweet-tart, semi-tender New Ohio variety. An improved PaulaRed type. Early August Eating, cooking Redfree Mildly tart and crunchy PRI release, 1981. Early-mid August Eating Sansa Sweet, crunchy, juicy Japan, 1988. Akane x Gala. Mid August Eating Ginger Gold G. Delicious type, tangier G Delicious seedling found in Virginia, late 1960s. Mid August All-purpose Zestar! Sweet-tart, crunchy, juicy U Minn, 1999. State Fair x MN 1691. Mid August Eating, cooking St Edmund's Pippin Juicy, crisp, rich flavor From Bury St Edmunds, 1870. Mid August Eating, cider Chenango Strawberry Mildly tart, berry flavors 1850s, Chenango County, NY Mid August Eating, cooking Summer Rambo Juicy, tart, aromatic 16th century, Rambure, France. Mid-late August Eating, sauce Honeycrisp Sweet, very crunchy, juicy U Minn, 1991. Unknown parentage. Late Aug.-early Sept. Eating Burgundy Tart, crisp 1974, from NY state Late Aug.-early Sept. All-purpose Blondee Sweet, crunchy, juicy New Ohio apple. Related to Gala. Late Aug.-early Sept. Eating Gala Sweet, crisp New Zealand, 1934. Golden Delicious x Cox Orange. Late Aug.-early Sept. Eating Swiss Gourmet Sweet-tart, juicy Switzerland. Golden x Idared. Late Aug.-early Sept. All-purpose Golden Supreme Sweet, Golden Delcious type Idaho, 1960. Golden Delicious seedling Early September Eating, cooking Pink Pearl Sweet-tart, bright pink flesh California, 1944, developed from Surprise Early September All-purpose Autumn Crisp Juicy, slow to brown Golden Delicious x Monroe. -

Bramble Volume 21, Issue 3
VOLUME 21, ISSUE 3 THE BRAMBLE AUTUMN, 2005 THE NEWSLETTER OF THE NORTH AMERICAN BRAMBLE GROWERS ASSOCIATION, INC. Request for Proposals ***** NABGA Annual Meeting & Conference ***** The North American Bramble Growers January 5-6, 2006 – Savannah, Georgia Research Foundation (NABGRF) is Our 2006 Annual Meeting will be held in association with the Georgia Fruit and seeking proposals for bramble research Vegetable Growers Association’s Southeast Fruit and Vegetable Conference (SFVC) in for the year 2006. Since 1999, NABGRF Savannah, Georgia. We hope to see you there! Watch your mailbox and e-mail for has funded a total of 26 proposals, registration details and accommodations information. totaling $50,146. To register for NABGA’s meeting, you will simply register for the SFVC. This All bramble proposals will be conference has a very large trade show and extensive sessions on blueberries, peaches, considered, however preference will be and vegetable crops January 6-8. Fees are very reasonable and both one-day and three- given to proposals related to: day registrations are available. The North American Strawberry Growers Association • cultivar development and testing (NASGA) will be meeting here (as the “North American Berry Conference”), on • pest management strategies January 4-6, with educational sessions on the 4th, a tour on January 5, and general • cultural management strategies to im- sessions the morning of the 6th. A forum on the National Berry Crops Initiative prove yield, quality and profitability Strategic Plan for the Berry Industry (see pages 8-9) is planned for Saturday, January • identification of beneficial com- 7. This concentration and combination of oppportunities is well worth the trip. -

Investigation of Wild Species Potential to Increase Genetic Diversity Useful for Apple Breeding
UDC 575.630 DOI: 10.2298/GENSR1503993D Original scientific paper INVESTIGATION OF WILD SPECIES POTENTIAL TO INCREASE GENETIC DIVERSITY USEFUL FOR APPLE BREEDING Catalina DAN1, Adriana F. SESTRAS1,*, Calin BOZDOG1,2, Radu E. SESTRAS1 1University of Agricultural Sciences and Veterinary Medicine, Cluj-Napoca, Romania 2Fruit Research Station, Cluj-Napoca, Romania Dan C., A. F. Sestras, C. Bozdog, R.E. Sestras (2015): Investigation of wild species potential to increase genetic diversity useful for apple breeding - Genetika, Vol 47, No. 3, 993-1011. The potential of testing new apple cultivars and the possibility to induce valuable traits is directly dependent on the availability of sufficient genetic diversity, while apple breeding has narrowed the genetic ground of commercial cultivars. Wild species were studied in regard to their influence upon progenies and their capacity to enlarge apple genetic diversity. The interspecific seedlings were framed in five biparental mating (paired crosses), in which Malus species were crossed with different cultivars, obtaining half-sib families. The number of F1 progenies per combination varied from 31 (Cluj 218/2 × M. floribunda) up to 142 (Reinette Baumann × M. floribunda), with a total of 1650 hybrids F1. The influences upon vigour and juvenile period and possible correlation among fruit size and taste were analyzed. Juvenile period varied from 6.00 (M. zumi × Jonathan) to 9.31 years (Cluj 218/2 × M. floribunda). Data based on correlation coefficient illustrated that the fructification year was not influenced by the vigour of trees. The highest value of correlation for fruit’s size and taste was obtained among M. coronaria hybrids. This result might suggest that once the fruit are larger, there is a high chance the taste is also more appreciative and fruit quality for mouth feels increase. -

Apples: Organic Production Guide
A project of the National Center for Appropriate Technology 1-800-346-9140 • www.attra.ncat.org Apples: Organic Production Guide By Tammy Hinman This publication provides information on organic apple production from recent research and producer and Guy Ames, NCAT experience. Many aspects of apple production are the same whether the grower uses low-spray, organic, Agriculture Specialists or conventional management. Accordingly, this publication focuses on the aspects that differ from Published nonorganic practices—primarily pest and disease control, marketing, and economics. (Information on March 2011 organic weed control and fertility management in orchards is presented in a separate ATTRA publica- © NCAT tion, Tree Fruits: Organic Production Overview.) This publication introduces the major apple insect pests IP020 and diseases and the most effective organic management methods. It also includes farmer profiles of working orchards and a section dealing with economic and marketing considerations. There is an exten- sive list of resources for information and supplies and an appendix on disease-resistant apple varieties. Contents Introduction ......................1 Geographical Factors Affecting Disease and Pest Management ...........3 Insect and Mite Pests .....3 Insect IPM in Apples - Kaolin Clay ........6 Diseases ........................... 14 Mammal and Bird Pests .........................20 Thinning ..........................20 Weed and Orchard Floor Management ......20 Economics and Marketing ........................22 Conclusion -

Cosmic Crisp's Growth Is out of This World
- Advertisement - Cosmic Crisp's growth is out of this world 1 / 2 April 26, 2021 After a record-breaking sophomore year that hasn't finished, the Cosmic Crisp apple’s growth trajectory indicates it will launch to the top of the apple chart in no time. Nielsen data show monumental growth, reaching No. 11 in sales dollars in March, and No. 14 over the 52 weeks ending March 27. If retailers do not have a Cosmic Crisp apple program in place now, they are missing out on very important sales and customer expectations. “Cosmic Crisp volume is planned to more than double this fall, and double again for the 2022 harvest. This volume will rival current core variety volume, displacing mature varieties that are being replaced with higher color strains and higher flavor varieties,” said Catherine Gipe-Stewart, communications manager. “Cosmic Crisp is rising so quickly, it has potential to become a top-five variety at this time next year.” Nielsen data show a 595 percent increase in dollars and a 720 percent in volume over the four weeks ending March 27. In March alone, Cosmic Crisp earned the 11th spot with $4.3 million in sales and 1.8 million pounds, at an average price of $2.52 per pound, right in line with Honeycrisp at $2.55 per pound. Looking at a yearly perspective (last 52 weeks), Cosmic Crisp has earned $20 million in sales, with 7.7 million pounds. “Historically, apples like Cosmic Crisp take root in the Pacific region, and spread eastward like wildfire,” said Gipe-Stewart. -

Survey of Apple Clones in the United States
Historic, archived document Do not assume content reflects current scientific knowledge, policies, or practices. 5 ARS 34-37-1 May 1963 A Survey of Apple Clones in the United States u. S. DFPT. OF AGRffini r U>2 4 L964 Agricultural Research Service U.S. DEPARTMENT OF AGRICULTURE PREFACE This publication reports on surveys of the deciduous fruit and nut clones being maintained at the Federal and State experiment stations in the United States. It will b- published in three c parts: I. Apples, II. Stone Fruit. , UI, Pears, Nuts, and Other Fruits. This survey was conducted at the request of the National Coor- dinating Committee on New Crops. Its purpose is to obtain an indication of the volume of material that would be involved in establishing clonal germ plasm repositories for the use of fruit breeders throughout the country. ACKNOWLEDGMENT Gratitude is expressed for the assistance of H. F. Winters of the New Crops Research Branch, Crops Research Division, Agricultural Research Service, under whose direction the questionnaire was designed and initial distribution made. The author also acknowledges the work of D. D. Dolan, W. R. Langford, W. H. Skrdla, and L. A. Mullen, coordinators of the New Crops Regional Cooperative Program, through whom the data used in this survey were obtained from the State experiment stations. Finally, it is recognized that much extracurricular work was expended by the various experiment stations in completing the questionnaires. : CONTENTS Introduction 1 Germany 298 Key to reporting stations. „ . 4 Soviet Union . 302 Abbreviations used in descriptions .... 6 Sweden . 303 Sports United States selections 304 Baldwin. -

Handling of Apple Transport Techniques and Efficiency Vibration, Damage and Bruising Texture, Firmness and Quality
Centre of Excellence AGROPHYSICS for Applied Physics in Sustainable Agriculture Handling of Apple transport techniques and efficiency vibration, damage and bruising texture, firmness and quality Bohdan Dobrzañski, jr. Jacek Rabcewicz Rafa³ Rybczyñski B. Dobrzañski Institute of Agrophysics Polish Academy of Sciences Centre of Excellence AGROPHYSICS for Applied Physics in Sustainable Agriculture Handling of Apple transport techniques and efficiency vibration, damage and bruising texture, firmness and quality Bohdan Dobrzañski, jr. Jacek Rabcewicz Rafa³ Rybczyñski B. Dobrzañski Institute of Agrophysics Polish Academy of Sciences PUBLISHED BY: B. DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES ACTIVITIES OF WP9 IN THE CENTRE OF EXCELLENCE AGROPHYSICS CONTRACT NO: QLAM-2001-00428 CENTRE OF EXCELLENCE FOR APPLIED PHYSICS IN SUSTAINABLE AGRICULTURE WITH THE th ACRONYM AGROPHYSICS IS FOUNDED UNDER 5 EU FRAMEWORK FOR RESEARCH, TECHNOLOGICAL DEVELOPMENT AND DEMONSTRATION ACTIVITIES GENERAL SUPERVISOR OF THE CENTRE: PROF. DR. RYSZARD T. WALCZAK, MEMBER OF POLISH ACADEMY OF SCIENCES PROJECT COORDINATOR: DR. ENG. ANDRZEJ STĘPNIEWSKI WP9: PHYSICAL METHODS OF EVALUATION OF FRUIT AND VEGETABLE QUALITY LEADER OF WP9: PROF. DR. ENG. BOHDAN DOBRZAŃSKI, JR. REVIEWED BY PROF. DR. ENG. JÓZEF KOWALCZUK TRANSLATED (EXCEPT CHAPTERS: 1, 2, 6-9) BY M.SC. TOMASZ BYLICA THE RESULTS OF STUDY PRESENTED IN THE MONOGRAPH ARE SUPPORTED BY: THE STATE COMMITTEE FOR SCIENTIFIC RESEARCH UNDER GRANT NO. 5 P06F 012 19 AND ORDERED PROJECT NO. PBZ-51-02 RESEARCH INSTITUTE OF POMOLOGY AND FLORICULTURE B. DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES ©Copyright by BOHDAN DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES LUBLIN 2006 ISBN 83-89969-55-6 ST 1 EDITION - ISBN 83-89969-55-6 (IN ENGLISH) 180 COPIES, PRINTED SHEETS (16.8) PRINTED ON ACID-FREE PAPER IN POLAND BY: ALF-GRAF, UL. -
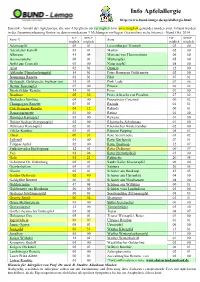
02 Apfelallergie Sortenliste Allgemein
Info Apfelallergie http://www.bund-lemgo.de/apfelallergie.html Statistik - Anzahl der Apfelsorten, die von Allergikern als verträglich bzw. unverträglich gemeldet worden sind. Erfasst werden in der Zusammenfassung Sorten zu denen mindestens 3 Meldungen vorliegen. Gesamtliste siehe Internet - Stand Okt. 2014 ver- unver- ver- unver- Sorte *) Sorte träglich träglich träglich träglich Adamsapfel 05 01 Luxemburger Triumph 03 00 Adersleber Kalvill 03 01 Martini 02 00 Alkmene 41 04 Minister von Hammerstein 06 00 Ananasrenette 06 01 Mutterapfel 05 00 Apfel aus Croncels 03 00 Notarisapfel 08 00 Arlet 02 01 Ontario 12 00 Altländer Pfannkuchenapfel 14 01 Peter Heusgens Goldrenette 02 00 Baumanns Renette 01 01 Pilot 07 01 Berlepsch, Goldrenette Freiherr von 35 03 Pink Lady 07 06 Berner Rosenapfel 07 00 Pinova 06 02 Biesterfelder Renette 14 01 Piros 01 00 Braeburn 05 32 Prinz Albrecht von Preußen 27 02 Brettacher Sämling 04 00 Purpurroter Cousinot 00 02 Champagner Renette 07 03 Reanda 01 01 Cox Orangen-Renette 04 12 Relinda 00 01 Damasonrenette 03 00 Retina 01 00 Danziger Kantapfel 05 00 Rewena 01 00 Doktor Seeligs Orangenapfel 02 00 Rheinische Schafsnase 01 00 Dülmener Rosenapfel 02 01 Rheinischer Winterrambur 02 00 Eifeler Rambur 03 01 Ripston Pepping 05 01 Elstar 09 31 Rote Sternrenette 03 02 Fallstaff 01 00 Roter Berlepsch 07 01 Filippas Apfel 02 00 Roter Boskoop 32 07 Finkenwerder Herbstprinz 12 01 Roter Delicious 00 07 Fuji 01 06 Roter Herbstkalvill 03 00 Gala 01 21 Rubinette 20 04 Geheimrat Dr. Oldenburg 02 03 Sankt Galler Klosterapfel 06 00