(12) Patent Application Publication (10) Pub. No.: US 2016/0220593 A1 ANASTASSOV Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GRAS Notice 789 for Erythritol
GRAS Notice (GRN) No. 789 https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory. Toi• Strategies ~~~~G~~[)) JUN 7 20'8 Innovative solutions Sound science OFFICE OF FOOD ADDITIVE SAFE1Y June 5, 2018 Dr. Dennis Keefe Director, Division of Biotechnology and GRAS Notice Review Office of Food Additive Safety (HFS-200) Center for Food Safety and Applied Nutrition Food and Drug Administration 5100 Paint Branch Parkway College Park, MD 20740-3835 Subject: GRAS Notification - Erythritol Dear Dr. Keefe: On behalf of Cargill, Incorporated, ToxStrategies, Inc. (its agent) is submitting, for FDA review, a copy of the GRAS notification as required. The enclosed document provides notice of a claim that the food ingredient, erythritol, described in the enclosed notification is exempt from the premarket approval requirement of the Federal Food, Drug, and Cosmetic Act because it has been determined to be generally recognized as safe (GRAS), based on scientific procedures, for addition to food. If you have any questions or require additional information, please do not hesitate to contact me at 630-352-0303, or [email protected]. Sincerely, (b) (6) Donald F. Schmitt, M.P.H. Senior Managing Scientist ToxStrategies, Inc., 931 W. 75th St. , Suite 137, PMB 263, Naperville, IL 60565 1 Office (630) 352-0303 • www.toxstrategies.com GRAS Determination of Erythritol for Use in Human Food JUNES,2018 Innovative solutions s ,..,.,',--.r-.r--.r--. OFFICE OF FOOD ADDITIVE SAFE1Y GRAS Determination of Erythritol for Use in Human Food SUBMITTED BY: Cargill, Incorporated 15407 McGinty Road West Wayzata, MN 55391 SUBMITTED TO: U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Food Additive Safety HFS-200 5100 Paint Branch Parkway College Park MD 20740-3835 CONTACT FOR TECHNICAL OR OTIIER INFORMATION Donald F. -

“Polyols: a Primer for Dietetic Professionals” Is a Self-Study
1 “Polyols: A primer for dietetic professionals” is a self-study module produced by the Calorie Control Council, an accredited provider of continuing professional education (CPE) for dietetic professionals by the Commission on Dietetic Registration. It provides one hour of level 1 CPE credit for dietetic professionals. The full text of the module is in the notes section of each page, and is accompanied by summary points and/or visuals in the box at the top of the page. Directions for obtaining CPE are provided at the end of the module. 2 After completing this module, dietetic professionals will be able to: • Define polyols. • Identify the various types of polyols found in foods. • Understand the uses and health effects of polyols in foods. • Counsel clients on how to incorporate polyols into an overall healthful eating pattern. 3 4 Polyols are carbohydrates that are hydrogenated, meaning that a hydroxyl group replaces the aldehyde or ketone group found on sugars. Hydrogenated monosaccharides include erythritol, xylitol, sorbitol, and mannitol. Hydrogenated disaccharides include lactitol, isomalt, and maltitol. And hydrogenated starch hydrolysates (HSH), or polyglycitols (a wide range of corn syrups and maltodextrins), are formed from polysaccharides (Grabitske and Slavin 2008). 5 Nearly 54 percent of Americans are trying to lose weight, more than ever before. Increasingly, they are turning toward no- and low-sugar, and reduced calorie, foods and beverages to help them achieve their weight loss goals (78% of Americans who are trying to lose weight) (CCC 2010). Polyols, found in many of these foods, are becoming a subject of more interest. 6 They are incompletely digested , therefore are sometimes referred to as “low- digestible carbohydrates.” Polyols are not calorie free, as there is some degree of digestion and absorption of the carbohydrate. -

Basics of Kraft Pulping
Lignin Wood is composed of many chemical components, primarily extractives, carbohydrates, and lignin, which are distributed nonuniformly as the result of anatomical structure. Lignin is derived from the Latin term lignum, which means wood.1 Anselme Payen (1838) was the first to recognize the composite nature of wood and referred to a carbon- rich substance as the “encrusting material” which embedded cellulose in the wood. Schulze (1865) later defined this encrusting material as lignin. Lignin has been described as a random, three-dimensional network polymer comprised of variously linked phenylpropane units.2 Lignin is the second most abundant biological material on the planet, exceeded only by cellulose and hemicellulose, and comprises 15-25% of the dry weight of woody plants. This macromolecule plays a vital role in providing mechanical support to bind plant fibers together. Lignin also decreases the permeation of water through the cell walls of the xylem, thereby playing an intricate role in the transport of water and nutrients. Finally, lignin plays an important function in a plant’s natural defense against degradation by impeding penetration of destructive enzymes through the cell wall. Although lignin is necessary to trees, it is undesirable in most chemical papermaking fibers and is removed by pulping and bleaching processes. 1.1.1 Biosynthesis Plant lignins can be broadly divided into three classes: softwood (gymnosperm), hardwood (angiosperm) and grass or annual plant (graminaceous) lignin.3 Three different phenylpropane units, or monolignols, are responsible for lignin biosynthesis.4 Guaiacyl lignin is composed principally of coniferyl alcohol units, while guaiacyl-syringyl lignin contains monomeric units from coniferyl and sinapyl alcohol. -
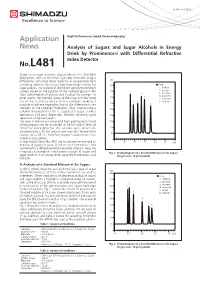
Analysis of Sugars and Sugar Alcohols in Energy Drink by Prominence-I with Differential Refreactive Index Detector
LAAN-A-LC-E258 Application High Performance Liquid Chromatography News Analysis of Sugars and Sugar Alcohols in Energy Drink by Prominence-i with Differential Refractive Index Detector No.L481 Sugars and sugar alcohols display almost no ultraviolet absorption, and are therefore typically detected using a differential refractive index detector or evaporative light uRI scattering detector. By using a ligand exchange column for 80 ■ Peaks sugar analysis, it is possible to distinguish among the different 1. maltose 70 2. glucose isomers based on the position of the hydroxyl group in the 1 4 3. fructose chair conformation of glucose and fructose for example. In 4. erythritol 60 5 other words, the hydroxyl group of the sugar and the metal 5. mannitol ion of the stationary phase form a complex, making it 2 3 6. sorbitol possible to achieve separation due to the difference in the 50 6 strength of the complex formation. Also, maintaining a 40 column temperature of 80 °C suppresses sugar anomer separation and peak dispersion, thereby achieving good 30 separation of adjacent peaks. The new Prominence-i integrated high-performance liquid 20 chromatograph can be connected to the RID-20A differential refractive index detector. The column oven, which can 10 accommodate a 30 cm column and maintain temperature 0 control up to 85 °C, therefore supports applications that require a long column. In Application News No. 467, we introduced an example of 0 5 10 15 20 25 min analysis of sugars in juice, in which the Prominence-i was connected to a differential refractive index detector. Here, we introduce an example of simultaneous analysis of sugars and Fig. -

Dental and Metabolic Effects of Lactitol in the Diet of Laboratory Rats
Downloaded from British Journal of Nutrition (1989), 61, 17-24 17 https://www.cambridge.org/core Dental and metabolic effects of lactitol in the diet of labolratory rats BY T. H. GRENBY AND A. PHILLIPS Department of Oral Medicine and Pathology, United Medical and Dental Schools, Guy’s Hospital, London SEI 9RT . IP address: (Received 17 May 1988 - Accepted 30 August 1988) 170.106.35.93 I. Because so little is known about the properties of lactitol as a possible alternative bulk sweetener to sucrose, it was tested in two large-scale experiments in laboratory rats. Matched groups of caries-active Osborne-Mendel rats were fed.on uniform diets containing lactitol and compared with a sucrose control in both experiments, plus a xylitol control in the first experiment. , on 2. In the early stages of the experiments weight gains and food utilization were better on the Sucrose than on 26 Sep 2021 at 05:12:03 the lactitol regimens. Body-fat storage was higher on the sucrose than on the polyol regimens. 3. At the end of 8 weeks the mandibular molars were examined for dental plaque accumulation and dental caries. The dental caries scores when 160 g sucrose/kg in the diet was replaced by lactitol were lower by a highly significant margin, bringing them down to the same low level as those on a 160 g xylitol/kg regimen. 4. Testing lactitol in a manufactured food product, shortbread biscuits, in comparison with ordinary sucrose biscuits, showed differences in plaque scores (significant) and caries levels (highly significant), with 60 % fewer lesions on the lactitol regimen. -

United States Patent Office
United States Patentaw Office ... ber of ether groupings on each molecule. These mix tures, however, if they analyze as containing an average 2,978,421. number of ether groups per molecule greater than one, NITRILE COPOLYMERS AND METHOD OF are capable of producing the insoluble nitrile polymers PREPARING SAME of this invention. Since the efficiency of the polyether cross-linking agent increases with the number of poten John A. Holloway, Cleveland, Ohio, assignor to The tially polymerizable groups on the molecule, it is much B. F. Goodrich Company, New York, N.Y., a corpo preferred to utilize polyethers containing an average of ration of New York - two or more alkenyl ether groupings per molecule. The No Drawing. Filed June 9, 1958, Ser. No. 740,566. 10 polyvinyl polyethers of the polyhydric alcohols within the above broad class are produced by reacting acetylene 9 Claims. (C. 260-17.4) with the polyhydric alcohol (or an alcoholate thereof) in a Reppe-type vinylation synthesis. The polycrotyl ethers of the polyhydric alcohols are also useful although they This invention relates to cross-linked alpha-beta ole 15 do not contain a terminal CHCC group. - finically unsaturated nitrile polymers and more particu Illustrative polyhydric alcohols of the above-described larly pertains to copolymers of alpha-beta olefinically class that may be utilized in the preparation of the poly. unsaturated nitriles and polyalkenyl polyethers of poly alkenyl polyether, cross-linking agent include the butane hydric alcohols and methods for their preparation. triols such as 1,2,3-butane triol, 2,3,4-trihydroxybutyric An object of this invention is the provision of insoluble, 20 acid, the aldotetroses such as erythrose and threose, keto cross-linked polynitriles which are capable of thickening tetroses such as erythrulose; the aldopentoses such as certain non-aqueous polar solvents. -

Polyols Have a Variety of Functional Properties That Make Them Useful Alternatives to Sugars in Applications Including Baked Goods
Polyols have a variety of functional properties that make them useful alternatives to sugars in applications including baked goods. Photo © iStockphoto.com/Synergee pg 22 09.12 • www.ift.org BY LYN NABORS and THERESA HEDRICK SUGAR REDUCTION WITH Polyols Polyols are in a unique position to assist with reduced-sugar or sugar-free reformulations since they can reduce calories and complement sugar’s functionality. ugar reduction will be an important goal over the of the product’s original characteristics may still be main- next few years as consumers, government, and in- tained with the replacement of those sugars by polyols. Sdustry alike have expressed interest in lower-calorie In addition, excellent, good-tasting sugar-free products and lower-sugar foods. The 2010 Dietary Guidelines for can be developed by using polyols. Polyols are in a unique Americans put a strong emphasis on consuming fewer position to assist with reduced-sugar or sugar-free refor- calories and reducing intake of added sugars. The In- mulations; since they are only partially digested and ab- stitute of Medicine (IOM) held a public workshop in sorbed, they can reduce calories and complement sugar’s November 2010 to discuss ways the food industry can functionality. Polyols provide the same bulk as sugars and use contemporary and innovative food processing tech- other carbohydrates. Additionally, polyols have a clean, nologies to reduce calorie intake in an effort to reduce sweet taste, which is important since consumers are not and prevent obesity, and in October 2011 recommended likely to sacrifice taste for perceived health benefits. Poly- front-of-package labeling that includes rating the product ols have a host of other functional properties that make based on added sugars content. -

Sweet Sensations by Judie Bizzozero | Senior Editor
[Confections] July 2015 Sweet Sensations By Judie Bizzozero | Senior Editor By R.J. Foster, Contributing Editor For many, terms like “reduced-sugar” or “sugar-free” do not go with the word “candy.” And yet, the confectionery industry is facing growing demand for treats that offer the taste people have grown to love without the adverse health effects they’re looking to avoid. Thankfully, there is a growing palette of ingredients from which candy makers can paint a new picture of sweetness that will be appreciated by the even most discerning of confectionery critics. SUGAR ALCOHOLS Also referred to as polyols, sugar alcohols are a common ingredient in reduced-sugar and sugar-free applications, especially confections. Funny thing, they’re not sugars or alcohols. Carbohydrate chains composed of monomeric, dimeric and polymeric units, polyols resemble both sugars and alcohols, but do not contain an ethanol molecule. All but two sugar alcohols are less sweet than sugar. Being only partially digestible, though, replacing a portion of a formulation’s sugar with a sugar alcohol reduces total calories without losing bulk (which can occur when replacing sugar with high-intensity sweeteners). Unique flavoring, texturizing and moisture-controlling effects also make polyols well-suited for confectionery products. Two very common and very similar monomeric polyols are sorbitol and mannitol. Present in a variety of fruits and vegetables, both are derived from products of cornstarch hydrolysis. Sorbitol is made via hydrogenation of glucose, which is why sorbitol is sometimes referred to as glucitol. Mannitol is created when fructose hydrogenation converts fructose into mannose, for which the final product, mannitol, is named. -

LIQUID-VAPOR EQUILIBRIUM THERMODYNAMICS of the FUSEL OIL: a CASE STUDY Jéssyka Jennifer Miranda Corrêa¹, Edilailsa Januário
LIQUID-VAPOR EQUILIBRIUM THERMODYNAMICS OF THE FUSEL OIL: A CASE STUDY Jéssyka Jennifer Miranda Corrêa¹, Edilailsa Januário de Melo², José Izaquiel Santos da Silva³ ¹Graduating in Chemical Engineering from the Federal University of the Jequitinhonha and Mucuri Valleys, Diamantina - MG, Brazil. ²PhD student in Chemical Engineering, College of Chemical Engineering - State University of Campinas - SP, Brazill. ³Professor at the Federal University of the Jequitinhonha and Mucuri Valleys, Diamantina - MG, Brazil. E-mail for contact: [email protected] Recebido em: 06/04/2019 – Aprovado em: 10/06/2019 – Publicado em: 30/06/2019 DOI: 10.18677/EnciBio_2019A183 ABSTRACT Fusel oil is a by-product of the process of recovering hydrated ethanol, consisting of a mixture of superior alcohols, ethanol, water, among other components. Its commercial interest is mainly due to the presence of isoamyl alcohol, one of the raw materials used in the synthesis of esters, which compounds are relevant for the chemical industry. The recovery of this superior alcohol involves unit operations based on phase equilibrium, which for multicomponent systems can be studied in software that simulates the operation of equipment, in order to present predictions of interactions between its components, reducing errors, time and costs of design. In the industrial, academic and scientific fields, there are numerous challenges regarding the design of separation processes and the monitoring of mixing effects in typical operations, and the Aspen Plus process simulation and optimization platform excels in solving these issues. The current study aims to predict the Liquid-Vapor Equilibrium of the fusel oil, which was considered as a mixture of five components, from the behavioral analysis of each of the binaries that compose it. -

Biovalorisation of Crude Glycerol and Xylose Into Xylitol by Oleaginous Yeast Yarrowia Lipolytica
Biovalorisation of crude glycerol and xylose into xylitol by oleaginous yeast Yarrowia lipolytica Ashish Prabhu Craneld University Dominic J Thomas Craneld University Rodrigo Ledesma- Amaro Imperial College London Gary A Leeke University of Birmingham Angel Medina Vaya Craneld University Carol Verheecke-Vaessen Craneld University Frederic Coulon Craneld University Vinod Kumar ( [email protected] ) Craneld University https://orcid.org/0000-0001-8967-6119 Research Keywords: Glycerol, Xylose, Yarrowia lipolytica, Biotransformation, Xylitol Posted Date: March 26th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-19009/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at Microbial Cell Factories on June 3rd, 2020. See the published version at https://doi.org/10.1186/s12934-020-01378-1. Page 1/28 Abstract Background: Xylitol is a commercially important chemical with multiple applications in food and pharmaceutical industries. According to the US Department of Energy, xylitol is among the twelve platform chemicals that can be produced from biomass. The chemical method for xylitol synthesis is however expensive and energy intensive. In contrast, the biological route involving microbial cell factories offers a potential cost-effective alternative process. The bioprocess occurs under ambient conditions and makes use of biocatalysts which can be sourced from renewable carbon coming from a variety of cheap feedstocks classied as wastes. Result: In this study, biotransformation of xylose to xylitol was investigated using Yarrowia lipolytica an oleaginous yeast grown on glycerol/glucose screening of primary carbon source, media optimisation in shake ask, scale up in bioreactor and downstream processing of xylitol were carried out. -

Lactitol, Bulk Sweetener for Sugar Free and Reduced Calories Hard
Sugar Free Dental Properties Lactitol is noncariogenic. It is not fermented by the oral micro flora, so its consumption does not lead to Lactitol, Bulk Sweetener for the formation of acids that deminer- alize the tooth enamel. Also, the building up of tooth plaque is much less for lactitol-containing hard can- dies when compared to sugar. Its Sugar Free and Reduced noncariogenic properties have been shown in various clinical trials (Grenby and Desai, 1988; Grenby, 1989; Grenby and Phillips, 1989; Grenby et al., 1989; van der Calories Hard Candy Hoeven, 1986). REGULATORY ASPECTS A self-affirmation petition for the Generally Recognized as Safe status of lactitol, submitted by Purac, was his paper will discuss the prop- intense sweetener like aspartame or accepted for filing by the Food and Terties, regulatory aspects, and acesulfame-K. The taste, sweetening power, and Drug Administration in September applications of lactitol in hard can- 1993. The safety of lactitol has been dies. Lactitol is a disaccharide sugar profile of such sweetener combina- tions are very close to those of substantiated by numerous animal alcohol made from lactose by cat- and human studies. This safety alytic hydrogenation. sucrose. Its clean sweet taste allows a superb flavor release. research has been reviewed by sev- eral international authoritative bod- BENEFITS OF LACTITOL Reduced Calories ies (JEFCA, 1983; EEC, 1984). The Taste The FDA allows the use of a self- joint FAO/WHO Committee on Lactitol has a clean, sweet, sugar- determined value of 2.0 kilocalorie Food Additives has approved lacti- like taste without an aftertaste. The per gram for lactitol. -

IPOLYHYDRIC ALCOHOLS from WOOD July 1954
IPOLYHYDRIC ALCOHOLS FROM WOOD July 1954 DEC 221954 No. 1984 UNITED STATES DEPARTMENT OF AGRICULTURE --,LFOREST. SERVICE 40REST PRODUCTS LABORATORY÷Q-.---,Y) Madison 5,Wisconsin In Cooperation with the University of Wisconsin 1 POLYHYDRIC ALCOHOLS FROM WOOD-2 2— By J. A. HALL, Director Forest Products Laboratory, 3 Forest Service U. S. Department of Agriculture Wood-sugar solutions produced in the laboratory-scale pilot plant at the U. S. Forest Products Laboratory have been proven to be satis- factory for the production of industrial alcohol, feeding yeast, and wood 4 molasses for stock feed (14, 15, 16, P 17). ± As a continuation of the pro- gram of the Laboratory for developing chemical utilization processes for the wood residues resulting from normal logging operations, and lumber, veneer, and the other forest products manufacture, investiga- tions are in progress to convert wood sugar into merchantable products. Because of the versatility and greatly increased demand for polytlydric alcohols, a study of the production of these materials has been chosen. The alkyd resin industry in the United States has increased tenfold in the past few years and no evidence of decline has been noted. Recently sorbitol has been made available commercially by the catalytic hydro- genation of corn sugar (2). The annual capacity now is 75 million pounds. Sorbitol is widely used as a humectant and conditioner in tobacco, cello- phane, and other products (3). However, one of its fast growing uses is for alkyd resins, where it may replace up to 50 percent glycerine and 25 percent pentaerythritol. This is fortunate since the production of glycerine in the United States as a byproduct of the soap manufacturing 1Presented before the Food and Agriculture Organization of the United Nations, Expert Panel on the Chemistry of Wood, meeting in Stockholm, Sweden, July 27-28, 1953.