Report/Management Plan for District Staff And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calaby References
Abbott, I.J. (1974). Natural history of Curtis Island, Bass Strait. 5. Birds, with some notes on mammal trapping. Papers and Proceedings of the Royal Society of Tasmania 107: 171–74. General; Rodents; Abbott, I. (1978). Seabird islands No. 56 Michaelmas Island, King George Sound, Western Australia. Corella 2: 26–27. (Records rabbit and Rattus fuscipes). General; Rodents; Lagomorphs; Abbott, I. (1981). Seabird Islands No. 106 Mondrain Island, Archipelago of the Recherche, Western Australia. Corella 5: 60–61. (Records bush-rat and rock-wallaby). General; Rodents; Abbott, I. and Watson, J.R. (1978). The soils, flora, vegetation and vertebrate fauna of Chatham Island, Western Australia. Journal of the Royal Society of Western Australia 60: 65–70. (Only mammal is Rattus fuscipes). General; Rodents; Adams, D.B. (1980). Motivational systems of agonistic behaviour in muroid rodents: a comparative review and neural model. Aggressive Behavior 6: 295–346. Rodents; Ahern, L.D., Brown, P.R., Robertson, P. and Seebeck, J.H. (1985). Application of a taxon priority system to some Victorian vertebrate fauna. Fisheries and Wildlife Service, Victoria, Arthur Rylah Institute of Environmental Research Technical Report No. 32: 1–48. General; Marsupials; Bats; Rodents; Whales; Land Carnivores; Aitken, P. (1968). Observations on Notomys fuscus (Wood Jones) (Muridae-Pseudomyinae) with notes on a new synonym. South Australian Naturalist 43: 37–45. Rodents; Aitken, P.F. (1969). The mammals of the Flinders Ranges. Pp. 255–356 in Corbett, D.W.P. (ed.) The natural history of the Flinders Ranges. Libraries Board of South Australia : Adelaide. (Gives descriptions and notes on the echidna, marsupials, murids, and bats recorded for the Flinders Ranges; also deals with the introduced mammals, including the dingo). -
Mammals of Fleurieu Peninsula This List of Mammals of Fleurieu Peninsula Was Applied by the Late P.F
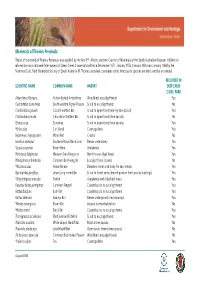
Mammals of Fleurieu Peninsula This list of mammals of Fleurieu Peninsula was applied by the late P.F. Aitken, onetime Curator of Mammals at the South Australian Museum. Additional information was obtained from surveys of Deep Creek Conservation Park in December 1971, January 1972, January 1980 and January 1984 by the Mammal Club, Field Naturalists Society of South Australia (R. Thomas, personal communication). Introduced species are indicated by an asterisk. RECORDED IN SCIENTIFIC NAME COMMON NAME HABITAT DEEP CREEK CONS. PARK Antechinus Flavipes Yellow-footed Antechinus Woodland, eucalypt forest Yes Cercartetus concinnus South western Pigmy Possum Scrub to eucalypt forest No Chalinolobus gouldii Gould's wattled Bat Scrub to open-forest (mainly tree spouts) Yes Chalinolobus moria Chocolate Wattled Bat Scrub to open-forest (tree spouts) No Eptesicus sp. Eptesicus Scrub to open-forest (tree spouts) Yes *Felis calus Cat (feral) Cosmopolitan Yes Hydromys chrysogaster Water Rat Creeks No Isoodon obesulus Southern Brown Bandicoot Dense understorey Yes *Lepus capensis Brown Hare Grasslands Yes Macropus fuliginosus Western Grey Kangaroo Heath to eucalypt forest Yes Miniopterus schreibersii Common Bent-wing Ba Eucalypt forest (caves) No *Mus musculus House Mouse Disturbed areas and early fire succession Yes Nyctophilus geoffroy Lesser Long-eared Bat Scrub to forest, semi cleared pasture (tree spouts, buildings) Yes *Oryctolagus cuniculus Rabbit Grasslands and disturbed areas Yes Pseudocheirus peregrinus Common Ringtail Coastal scrub to eucalypt -

Natural History of the Eutheria
FAUNA of AUSTRALIA 35. NATURAL HISTORY OF THE EUTHERIA P. J. JARMAN, A. K. LEE & L. S. HALL (with thanks for help to J.H. Calaby, G.M. McKay & M.M. Bryden) 1 35. NATURAL HISTORY OF THE EUTHERIA 2 35. NATURAL HISTORY OF THE EUTHERIA INTRODUCTION Unlike the Australian metatherian species which are all indigenous, terrestrial and non-flying, the eutherians now found in the continent are a mixture of indigenous and exotic species. Among the latter are some intentionally and some accidentally introduced species, and marine as well as terrestrial and flying as well as non-flying species are abundantly represented. All the habitats occupied by metatherians also are occupied by eutherians. Eutherians more than cover the metatherian weight range of 5 g–100 kg, but the largest terrestrial eutherians (which are introduced species) are an order of magnitude heavier than the largest extant metatherians. Before the arrival of dingoes 4000 years ago, however, none of the indigenous fully terrestrial eutherians weighed more than a kilogram, while most of the exotic species weigh more than that. The eutherians now represented in Australia are very diverse. They fall into major suites of species: Muridae; Chiroptera; marine mammals (whales, seals and dugong); introduced carnivores (Canidae and Felidae); introduced Leporidae (hares and rabbits); and introduced ungulates (Perissodactyla and Artiodactyla). In this chapter an attempt is made to compare and contrast the main features of the natural histories of these suites of species and, where appropriate, to comment on their resemblance to or difference from the metatherians. NATURAL HISTORY Ecology Diet. The native rodents are predominantly omnivorous. -

Ba3444 MAMMAL BOOKLET FINAL.Indd
Intot Obliv i The disappearing native mammals of northern Australia Compiled by James Fitzsimons Sarah Legge Barry Traill John Woinarski Into Oblivion? The disappearing native mammals of northern Australia 1 SUMMARY Since European settlement, the deepest loss of Australian biodiversity has been the spate of extinctions of endemic mammals. Historically, these losses occurred mostly in inland and in temperate parts of the country, and largely between 1890 and 1950. A new wave of extinctions is now threatening Australian mammals, this time in northern Australia. Many mammal species are in sharp decline across the north, even in extensive natural areas managed primarily for conservation. The main evidence of this decline comes consistently from two contrasting sources: robust scientifi c monitoring programs and more broad-scale Indigenous knowledge. The main drivers of the mammal decline in northern Australia include inappropriate fi re regimes (too much fi re) and predation by feral cats. Cane Toads are also implicated, particularly to the recent catastrophic decline of the Northern Quoll. Furthermore, some impacts are due to vegetation changes associated with the pastoral industry. Disease could also be a factor, but to date there is little evidence for or against it. Based on current trends, many native mammals will become extinct in northern Australia in the next 10-20 years, and even the largest and most iconic national parks in northern Australia will lose native mammal species. This problem needs to be solved. The fi rst step towards a solution is to recognise the problem, and this publication seeks to alert the Australian community and decision makers to this urgent issue. -

Karoo Bush Rat
Otomys unisulcatus – Karoo Bush Rat threats that could cause widespread population decline. However, there are potentially synergistic effects of climate change drying up wetlands and overgrazing/ browsing removing at least part of the plant food and cover that this species relies upon. Such effects on subpopulation trends and population distribution should be monitored. Regional population effects: This species is endemic to the assessment region. Its dispersal abilities are not well known. Subpopulations seem to be patchily distributed at the landscape level, according to the presence of favourable habitats. While it is likely that movements and possibly rescue effects exist between subpopulations, Emmanuel Do Linh San others might be physically and genetically isolated. Regional Red List status (2016) Least Concern Distribution National Red List status (2004) Least Concern This species occurs throughout the semi-arid Succulent Reasons for change No change Karoo and Nama-Karoo of South Africa (Monadjem et al. 2015), specifically in the Eastern, Northern and Western Global Red List status (2016) Least Concern Cape provinces, with some limited occurrence in the TOPS listing (NEMBA) (2007) None Fynbos Biome (Vermeulen & Nel 1988; Figure 1). It may marginally occur in southern Namibia but further surveys CITES listing None are required to confirm this. Regardless, the bulk of the Endemic Yes population occurs in South Africa. Kerley and Erasmus (1992) argued that the lodges built by this species are In southern Africa the Karoo Bush Rat vulnerable to destruction by fire. As a result, they is the only rodent that constructs and occupies hypothesised that this shelter-building strategy is only large, dome-shaped stick nests or “lodges”, viable in the absence of frequent burning, and therefore it generally at the base of bushes. -

Terrestrial Native Mammals of Western Australia
TERRESTRIALNATIVE MAMMALS OF WESTERNAUSTRALIA On a number of occasionswe have been asked what D as y ce r cus u ist ica ud q-Mul Aara are the marsupialsof W.A. or what is the scientiflcname Anlechinusfla.t,ipes Matdo given to a palticular animal whosecommon name only A n t ec h i nus ap i ca I i s-Dlbbler rs known. Antechinusr osemondae-Little Red Antechinus As a guide,the following list of62 speciesof marsupials A nteclt itus mqcdonneIlens is-Red-eared Antechi nus and 59 speciesof othersis publishedbelow. Antechinus ? b ilar n i-Halney' s Antechinus Antec h in us mqculatrJ-Pismv Antechinus N ingaui r idei-Ride's Nirfaui - MARSUPALIA Ningauirinealvi Ealev's-KimNinsaui Ptaiigole*fuilissima beiiey Planigale Macropodidae Plani gale tenuirostris-Narrow-nosed Planigate Megaleia rufa Red Kangaroo Smi nt hopsis mu rina-Common Dulnart Macropus robustus-Etro Smin t hop[is longicaudat.t-Long-tailed Dunnart M acr opus fu Ii g inos,s-Western Grey Kangaroo Sminthops is cras sicaudat a-F at-tailed Dunnart Macrcpus antilo nus Antilope Kangaroo S-nint hopsi s froggal//- Larapinla Macropu"^agi /rs Sandy Wallaby Stnintllopsirgranuli,oer -Whire-railed Dunnart Macrcpus rirra Brush Wallaby Sninthopsis hir t ipes-Hairy -footed Dunnart M acro ptrs eugenii-T ammar Sminthopsiso oldea-^f r oughton's Dunnart Set oni x brac ltyuru s-Quokka A ntec h inomys lanrger-Wuhl-Wuhl On y ch oga I ea Lng uife r a-Kar r abul M.yr nte c o b ius fasc ialrls-N umbat Ony c hogalea Iunq ta-W \rrur.g Notoryctidae Lagorchest es conspic i Ilat us,Spectacied Hare-Wallaby Notorlctes -

Fitz-Stirling 2007-2017 Ten-Year Evaluation Review
Fitz-Stirling 2007-2017 Ten-year Evaluation Review Feb / 2018 P a g e | 1 Acknowledgements: This report has benefited greatly from the discussion and guidance on content, presentation and editing by Annette Stewart, Clair Dougherty and Simon Smale. Their expert assistance is greatly appreciated. Volunteers have played a major and vital role in the monitoring and survey program over the past 5 years and I thank all of those involved. Special thanks go to Dr Sandra Gilfillan for her continuing dedication to the wallaby monitoring and research program. Volunteers Aaron Gove, who provided the bird data analysis and Richard Thomas, who provided the bat data analysis, have made a large contribution to this report and I thank them. I sincerely thank Bill and Jane Thompson who have regularly carried out all the pool monitoring for several years. Thanks also to Barry Heydenrych, Greening Australia, who provided restoration data. Funding to assist the monitoring program and UAV surveys during 2015 was gratefully received from South Coast NRM as part of the Australian Government funded ‘Restoring Gondwana’ program. Funding vital for wallaby monitoring and research was provided by the Diversicon Foundation. Citation: Sanders, A. (2018). Fitz-Stirling 2007-2017 ten-year evaluation review. Unpublished report for Bush Heritage Australia. P a g e | 2 Contents Overview of Fitz-Stirling Project ........................................................................................................ 6 This report evaluates our conservation impact ................................................................................. -

Rattus Fuscipes
MAMMALIAN SPECIES No. 298, pp. 1-8, 3 figs. Rattus fuscipes. By J. Mary Taylor and John H. Calaby Published 15 January 1988 by The American Society of Mammalogists Rattus juscipes (Waterhouse., 1839) Nasals overhang anterior faces of incisors and premaxillae on the relatively narrow rostrum. Tympanic bullae equal in length, or slight Bush Rat ly smaller than, alveolar length of M1-M3. A posterior cusp present Mus fuscipes Waterhouse, 1839:66. Neotype locality 4 mi. S. Mt. on m1 and m2 (Taylor and Horner, 1973a). Melville, Albany, Western Australia. Downloaded from https://academic.oup.com/mspecies/article/doi/10.2307/3503837/2600613 by guest on 01 October 2021 Mus greyii Gray, 1841:410. Lectotype locality South Australia. DISTRIBUTION. Rattus fuscipes is a mainland species; it Mus assimilis Gould, 1858:241. Lectotype locality Clarence River, is primarily coastal in distribution and also occupies offshore islands New South Wales. (Fig. 3). It occurs in southwestern Australia, from near Jurien Bay Rattus mondraineus Thomas, 1921:428. Type locality Mondrain to Israelite Bay and on the Abrolhos Islands farther north; discon Island, Archipelago of the Recherche, Western Australia. tinuously along southern and eastern regions from Eyre Peninsula, Rattus murrayi Thomas, 1923a:601. Type locality Pearson's Is South Australia, including offshore islands, to near Portland, Victoria; land, Investigator Group, South Australia. from Terang, Victoria, to near Rockhampton, Queensland; and from Rattus glauerti Thomas, 1926:308. Type locality East Wallaby Townsville to Cooktown in northern Queensland (Taylor and Horner, Island, Houtman's Abrolhos, Western Australia. 197 3a; Watts and Aslin, 1981). Although mainly a lowland species, R. -

Factors Influencing the Distribution of Bush Rats
Factors influencing the distribution of Bush rats Rattus fuscipes Bush rats (photo Wendy Gleen) Bush rat habitat (photo Wendy Kinsella) A thesis submitted for Master of Philosophy at the University of NSW 2014 by Wendy Kinsella The School of Biological, Earth and Environmental Science The University of New South Wales Sydney NSW, Australia Table of Contents Student Declaration ................................................................................................................. v Acknowledgement ................................................................................................................... vi List of Figures ......................................................................................................................... vii List of Tables ........................................................................................................................... ix Abstract .................................................................................................................................... xi Chapter 1 .................................................................................................................................. 1 General Introduction ............................................................................................................... 1 1.1 Habitat requirements for a small mammal ..................................................................... 1 1.2 Australia’s Urban Landscape .......................................................................................... -

Small Native Mammals Equals a Healthy Environment and Functioning Ecosystem
What should I do if I discover small mammals in my building? ENSURE AREAS WHERE FOOD IS KEPT OR CONSUMED ARE CLEAN. Check behind fridges, in cupboards and beneath dishwashers for food scraps or crumbs that may It is not uncommon to find our native attract small mammals. Ensure all food is stored in containers that prevent mammals living inside buildings. In access, and are kept in secure cupboards or pantries. winter when it is cold outside and there Block up any entrances, holes or gaps through which is little food around, ski lodges offer animals may be gaining entry to living areas. It may be both warmth and food. Most buildings impossible or impractical to prevent them getting into the roof, walls or beneath the building but blocking may seem secure and solid to us, their access into your living areas is possible. but offer plenty of gaps, holes and DO NOT USE SNAP TRAPS OR POISON BAITS. cracks that allow access to these tiny, Purchase a small mammal trap for $5 from Resort Management, a user friendly, reusable, catch and agile animals. Remember these are release trap. protected native animals that have lived Love and appreciate them. It is illegal to kill or distantly harmoniously in this area for thousands relocate them. Penalties apply for killing native wildlife. of years, long before development The presence of small native mammals equals a healthy environment and functioning ecosystem. commenced on the mountain. We - lirralirra.com Photo © Kim Wormald are all obliged to protect them. Antechinus agilis mtbuller.com.au Small Native Mammals Mt Buller Mt Stirling Resort Management Alpine Central Summit Road Mt Buller VIC 3723 Telephone 03 5777 6077 Email [email protected] Dusky Antechinus Antechinus swainsonii Antechinus Southern Bush Rat Broad Toothed Rat Antechinus are one of the most common animals at Mt Buller. -

On Mammalian Sperm Dimensions J
On mammalian sperm dimensions J. M. Cummins and P. F. Woodall Reproductive Biology Group, Department of Veterinary Anatomy, University of Queensland, St Lucia, Queensland4067, Australia Summary. Data on linear sperm dimensions in mammals are presented. There is infor- mation on a total of 284 species, representing 6\m=.\2%of all species; 17\m=.\2%of all genera and 49\m=.\2%of all families have some representation, with quantitative information missing only from the orders Dermoptera, Pholidota, Sirenia and Tubulidentata. In general, sperm size is inverse to body mass (except for the Chiroptera), so that the smallest known spermatozoa are amongst those of artiodactyls and the largest are amongst those of marsupials. Most variations are due to differences in the lengths of midpiece and principal piece, with head lengths relatively uniform throughout the mammals. Introduction There is increasing interest in comparative studies of gametes both from the phylogenetic viewpoint (Afzelius, 1983) and also in the analysis of the evolution of sexual reproduction and anisogamy (Bell, 1982; Parker, 1982). This work emerged as part of a review of the relationship between sperm size and body mass in mammals (Cummins, 1983), in which lack of space precluded the inclusion of raw data. In publishing this catalogue of sperm dimensions we wish to rectify this defect, and to provide a reference point for, and stimulus to, further quantitative work while obviating the need for laborious compilation of raw data. Some aspects of the material presented previously (Cummins, 1983) have been re-analysed in the light of new data. Materials and Methods This catalogue of sperm dimensions has been built up from cited measurements, from personal observations and from communication with other scientists. -

Rodents Fact Sheet
Rodents There are approximately 60 native species in Australia including the well-known Water Rat, Bush Rat and Stick-nest Rats. All Australian rodents belong to the single family, Muridae. Sewer Rat, Rattus norvegicus (introduced) The three pest species belong to the subfamily, Murinae. It is not uncommon for native species such as bush • Body length 240-millimetres, tail length 200-millimetres, rats and various species of mice to be found in people’s weight 300-grams. backyards, so it is imperative that you can identify the • Shaggy fur, colour varies from black to white, but is animal’s species before undertaking a control program. generally brown on the back and dirty white on belly, thick tail. Impact of rodents • Small ears, when pulled forward the ears just reach Rats and mice create food spoilage, contamination and the the eyes. transmission of diseases to humans. The most common • Found around human habitation, in particular wharves, disease transmitted by rats is Salmonellosis, which is warehouses, tunnels, drains and sewers. spread when food consumed by humans has been • Nocturnal by nature. Non-climbing, digs burrows. contaminated by excreta or saliva of rats. Feeds on meat (insects, prawns, birds, eggs, mice) Physical damage caused by gnawing can sometimes lead and scraps. to costly fires. Rodents’ habitats include holes and burrows House Mouse, Mus musculus (introduced) in the ground and any place where food and shelter are within reasonable range. Indoors, they may construct nests • Body length 75-millimetres, tail length 80-millimetres, in wall voids, in roof voids, under floors and even within weight 15-grams.