Lowland Woodland Fauna
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Managing Diversity in the Riverina Rice Fields—
Reconciling Farming with Wildlife —Managing diversity in the Riverina rice fields— RIRDC Publication No. 10/0007 RIRDCInnovation for rural Australia Reconciling Farming with Wildlife: Managing Biodiversity in the Riverina Rice Fields by J. Sean Doody, Christina M. Castellano, Will Osborne, Ben Corey and Sarah Ross April 2010 RIRDC Publication No 10/007 RIRDC Project No. PRJ-000687 © 2010 Rural Industries Research and Development Corporation. All rights reserved. ISBN 1 74151 983 7 ISSN 1440-6845 Reconciling Farming with Wildlife: Managing Biodiversity in the Riverina Rice Fields Publication No. 10/007 Project No. PRJ-000687 The information contained in this publication is intended for general use to assist public knowledge and discussion and to help improve the development of sustainable regions. You must not rely on any information contained in this publication without taking specialist advice relevant to your particular circumstances. While reasonable care has been taken in preparing this publication to ensure that information is true and correct, the Commonwealth of Australia gives no assurance as to the accuracy of any information in this publication. The Commonwealth of Australia, the Rural Industries Research and Development Corporation (RIRDC), the authors or contributors expressly disclaim, to the maximum extent permitted by law, all responsibility and liability to any person, arising directly or indirectly from any act or omission, or for any consequences of any such act or omission, made in reliance on the contents of this publication, whether or not caused by any negligence on the part of the Commonwealth of Australia, RIRDC, the authors or contributors. The Commonwealth of Australia does not necessarily endorse the views in this publication. -

Calaby References
Abbott, I.J. (1974). Natural history of Curtis Island, Bass Strait. 5. Birds, with some notes on mammal trapping. Papers and Proceedings of the Royal Society of Tasmania 107: 171–74. General; Rodents; Abbott, I. (1978). Seabird islands No. 56 Michaelmas Island, King George Sound, Western Australia. Corella 2: 26–27. (Records rabbit and Rattus fuscipes). General; Rodents; Lagomorphs; Abbott, I. (1981). Seabird Islands No. 106 Mondrain Island, Archipelago of the Recherche, Western Australia. Corella 5: 60–61. (Records bush-rat and rock-wallaby). General; Rodents; Abbott, I. and Watson, J.R. (1978). The soils, flora, vegetation and vertebrate fauna of Chatham Island, Western Australia. Journal of the Royal Society of Western Australia 60: 65–70. (Only mammal is Rattus fuscipes). General; Rodents; Adams, D.B. (1980). Motivational systems of agonistic behaviour in muroid rodents: a comparative review and neural model. Aggressive Behavior 6: 295–346. Rodents; Ahern, L.D., Brown, P.R., Robertson, P. and Seebeck, J.H. (1985). Application of a taxon priority system to some Victorian vertebrate fauna. Fisheries and Wildlife Service, Victoria, Arthur Rylah Institute of Environmental Research Technical Report No. 32: 1–48. General; Marsupials; Bats; Rodents; Whales; Land Carnivores; Aitken, P. (1968). Observations on Notomys fuscus (Wood Jones) (Muridae-Pseudomyinae) with notes on a new synonym. South Australian Naturalist 43: 37–45. Rodents; Aitken, P.F. (1969). The mammals of the Flinders Ranges. Pp. 255–356 in Corbett, D.W.P. (ed.) The natural history of the Flinders Ranges. Libraries Board of South Australia : Adelaide. (Gives descriptions and notes on the echidna, marsupials, murids, and bats recorded for the Flinders Ranges; also deals with the introduced mammals, including the dingo). -

An Intial Estimation of the Numbers and Identification of Extant Non
Answers Research Journal 8 (2015):171–186. www.answersingenesis.org/arj/v8/lizard-kinds-order-squamata.pdf $Q,QLWLDO(VWLPDWLRQRIWKH1XPEHUVDQG,GHQWLÀFDWLRQRI Extant Non-Snake/Non-Amphisbaenian Lizard Kinds: Order Squamata Tom Hennigan, Truett-McConnell College, Cleveland, Georgia. $EVWUDFW %LRV\VWHPDWLFVLVLQJUHDWÁX[WRGD\EHFDXVHRIWKHSOHWKRUDRIJHQHWLFUHVHDUFKZKLFKFRQWLQXDOO\ UHGHÀQHVKRZZHSHUFHLYHUHODWLRQVKLSVEHWZHHQRUJDQLVPV'HVSLWHWKHODUJHDPRXQWRIGDWDEHLQJ SXEOLVKHGWKHFKDOOHQJHLVKDYLQJHQRXJKNQRZOHGJHDERXWJHQHWLFVWRGUDZFRQFOXVLRQVUHJDUGLQJ WKHELRORJLFDOKLVWRU\RIRUJDQLVPVDQGWKHLUWD[RQRP\&RQVHTXHQWO\WKHELRV\VWHPDWLFVIRUPRVWWD[D LVLQJUHDWIOX[DQGQRWZLWKRXWFRQWURYHUV\E\SUDFWLWLRQHUVLQWKHILHOG7KHUHIRUHWKLVSUHOLPLQDU\SDSHU LVmeant to produce a current summary of lizard systematics, as it is understood today. It is meant to lay a JURXQGZRUNIRUFUHDWLRQV\VWHPDWLFVZLWKWKHJRDORIHVWLPDWLQJWKHQXPEHURIEDUDPLQVEURXJKWRQ WKH $UN %DVHG RQ WKH DQDO\VHV RI FXUUHQW PROHFXODU GDWD WD[RQRP\ K\EULGL]DWLRQ FDSDELOLW\ DQG VWDWLVWLFDO EDUDPLQRORJ\ RI H[WDQW RUJDQLVPV D WHQWDWLYH HVWLPDWH RI H[WDQW QRQVQDNH QRQ DPSKLVEDHQLDQOL]DUGNLQGVZHUHWDNHQRQERDUGWKH$UN,WLVKRSHGWKDWWKLVSDSHUZLOOHQFRXUDJH IXWXUHUHVHDUFKLQWRFUHDWLRQLVWELRV\VWHPDWLFV Keywords: $UN(QFRXQWHUELRV\VWHPDWLFVWD[RQRP\UHSWLOHVVTXDPDWDNLQGEDUDPLQRORJ\OL]DUG ,QWURGXFWLRQ today may change tomorrow, depending on the data Creation research is guided by God’s Word, which and assumptions about that data. For example, LVIRXQGDWLRQDOWRWKHVFLHQWLÀFPRGHOVWKDWDUHEXLOW naturalists assume randomness and universal 7KHELEOLFDODQGVFLHQWLÀFFKDOOHQJHLVWRLQYHVWLJDWH -

Adec Preview Generated PDF File
Rec. West. Aust. Mus., 1976,4 (2) THE GENUS MENETIA (LACERTILIA, SCINCIDAE) IN WESTERN AUSTRALIA G.M. STORR* [Received 1 July 1975. Accepted 1 October 1975. Published 30 September 1976.] ABSTRACT The Australian genus Menetia comprises at least five species, three of which occur in Western Australia, namely M. greyii Gray, M. maini novo and M. surda novo A lectotype is designated for M. greyii. INTRODUCTION Until recently all skinks with an immovable transparent lower eyelid were placed in Ablepharus. Fuhn (1969) broke up this polyphyletic assemblage, allotting the Australian species to nine groups, including the genus Menetia. Fuhn, and indeed all workers till now, regarded Menetia as monotypic. Greer (1974) believes that Menetia is derived from the genus Carlia. All the material used in this revision is lodged in the Western Australian Museum. Genus Menetia Gray Menetia Gray, 1845, 'Catalogue of the specimens of lizards in the collection ofthe British Museum', p.65. Type-species (by monotypy): M. greyii Gray. * Curator of Birds and Reptiles, W.A. Museum. 189 Diagnosis Very small, smooth, terrestrial skinks with lower eyelid immovable and bearing a large circular transparent disc incompletely surrounded by granules; digits 4 + 5; first supraocular long and narrow and obliqu~ly orientated. Distribution Most of Australia except the wettest and coolest regions. At least five species, three of them in Western Australia. Description Snout-vent length up to 38 mm. Tail fragile, 1.2-2.0 times as long as snout to vent. Nasals usually separated widely. No supranasals or postnasals. Prefrontals usually separated very narrowly. Frontal small, little if any larger than prefrontals. -

The Ecological Basis of Sensitivity of Brown Treecreepers to Habitat Fragmentation: a Preliminary Assessment
Biological Conservation 90 (1999) 13±20 The ecological basis of sensitivity of brown treecreepers to habitat fragmentation: a preliminary assessment Jerey R. Walters a,*, Hugh A. Ford b, Caren B. Cooper c aDepartment of Zoology, North Carolina State University, Raleigh, NC 27695-7617, USA bDepartment of Zoology, University of New England, Armidale, NSW 2351, Australia cDepartment of Biology, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061-0406, USA Received 4 April 1998; received in revised form 22 October 1998; accepted 5 January 1999 Abstract We attempted to identify the mechanisms responsible for adverse eects of habitat fragmentation on brown treecreepers (Cli- macteris picumnus) inhabiting eucalyptus woodland in northeastern New South Wales, Australia by comparing demography and foraging ecology of birds in highly fragmented and relatively unfragmented landscapes. In particular, we investigated three possi- bilities, disrupted dispersal due to patch isolation, reduced fecundity due to elevated nest predation, and reduced food availability due to habitat degradation. Nesting success was high in both highly fragmented and less fragmented habitat. Of ®rst nests, 88% were successful, and 60% of successful groups attempted a second brood. However, there were many more groups in the more fragmented habitat than in the less fragmented habitat that lacked a female for most or all of the breeding season, and thus did not attempt nesting (64% vs 13%). In both the more fragmented and the less fragmented habitat, both males and females spent about 70% of their time foraging and 65% of their foraging time on the ground. We reject reduced fecundity in fragmented habitat as an explanation of adverse eects of habitat fragmentation on brown treecreepers. -
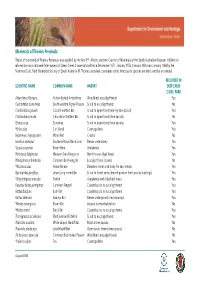
Mammals of Fleurieu Peninsula This List of Mammals of Fleurieu Peninsula Was Applied by the Late P.F
Mammals of Fleurieu Peninsula This list of mammals of Fleurieu Peninsula was applied by the late P.F. Aitken, onetime Curator of Mammals at the South Australian Museum. Additional information was obtained from surveys of Deep Creek Conservation Park in December 1971, January 1972, January 1980 and January 1984 by the Mammal Club, Field Naturalists Society of South Australia (R. Thomas, personal communication). Introduced species are indicated by an asterisk. RECORDED IN SCIENTIFIC NAME COMMON NAME HABITAT DEEP CREEK CONS. PARK Antechinus Flavipes Yellow-footed Antechinus Woodland, eucalypt forest Yes Cercartetus concinnus South western Pigmy Possum Scrub to eucalypt forest No Chalinolobus gouldii Gould's wattled Bat Scrub to open-forest (mainly tree spouts) Yes Chalinolobus moria Chocolate Wattled Bat Scrub to open-forest (tree spouts) No Eptesicus sp. Eptesicus Scrub to open-forest (tree spouts) Yes *Felis calus Cat (feral) Cosmopolitan Yes Hydromys chrysogaster Water Rat Creeks No Isoodon obesulus Southern Brown Bandicoot Dense understorey Yes *Lepus capensis Brown Hare Grasslands Yes Macropus fuliginosus Western Grey Kangaroo Heath to eucalypt forest Yes Miniopterus schreibersii Common Bent-wing Ba Eucalypt forest (caves) No *Mus musculus House Mouse Disturbed areas and early fire succession Yes Nyctophilus geoffroy Lesser Long-eared Bat Scrub to forest, semi cleared pasture (tree spouts, buildings) Yes *Oryctolagus cuniculus Rabbit Grasslands and disturbed areas Yes Pseudocheirus peregrinus Common Ringtail Coastal scrub to eucalypt -

Phylogeography of Finches and Sparrows
In: Animal Genetics ISBN: 978-1-60741-844-3 Editor: Leopold J. Rechi © 2009 Nova Science Publishers, Inc. Chapter 1 PHYLOGEOGRAPHY OF FINCHES AND SPARROWS Antonio Arnaiz-Villena*, Pablo Gomez-Prieto and Valentin Ruiz-del-Valle Department of Immunology, University Complutense, The Madrid Regional Blood Center, Madrid, Spain. ABSTRACT Fringillidae finches form a subfamily of songbirds (Passeriformes), which are presently distributed around the world. This subfamily includes canaries, goldfinches, greenfinches, rosefinches, and grosbeaks, among others. Molecular phylogenies obtained with mitochondrial DNA sequences show that these groups of finches are put together, but with some polytomies that have apparently evolved or radiated in parallel. The time of appearance on Earth of all studied groups is suggested to start after Middle Miocene Epoch, around 10 million years ago. Greenfinches (genus Carduelis) may have originated at Eurasian desert margins coming from Rhodopechys obsoleta (dessert finch) or an extinct pale plumage ancestor; it later acquired green plumage suitable for the greenfinch ecological niche, i.e.: woods. Multicolored Eurasian goldfinch (Carduelis carduelis) has a genetic extant ancestor, the green-feathered Carduelis citrinella (citril finch); this was thought to be a canary on phonotypical bases, but it is now included within goldfinches by our molecular genetics phylograms. Speciation events between citril finch and Eurasian goldfinch are related with the Mediterranean Messinian salinity crisis (5 million years ago). Linurgus olivaceus (oriole finch) is presently thriving in Equatorial Africa and was included in a separate genus (Linurgus) by itself on phenotypical bases. Our phylograms demonstrate that it is and old canary. Proposed genus Acanthis does not exist. Twite and linnet form a separate radiation from redpolls. -

Natural History of the Eutheria
FAUNA of AUSTRALIA 35. NATURAL HISTORY OF THE EUTHERIA P. J. JARMAN, A. K. LEE & L. S. HALL (with thanks for help to J.H. Calaby, G.M. McKay & M.M. Bryden) 1 35. NATURAL HISTORY OF THE EUTHERIA 2 35. NATURAL HISTORY OF THE EUTHERIA INTRODUCTION Unlike the Australian metatherian species which are all indigenous, terrestrial and non-flying, the eutherians now found in the continent are a mixture of indigenous and exotic species. Among the latter are some intentionally and some accidentally introduced species, and marine as well as terrestrial and flying as well as non-flying species are abundantly represented. All the habitats occupied by metatherians also are occupied by eutherians. Eutherians more than cover the metatherian weight range of 5 g–100 kg, but the largest terrestrial eutherians (which are introduced species) are an order of magnitude heavier than the largest extant metatherians. Before the arrival of dingoes 4000 years ago, however, none of the indigenous fully terrestrial eutherians weighed more than a kilogram, while most of the exotic species weigh more than that. The eutherians now represented in Australia are very diverse. They fall into major suites of species: Muridae; Chiroptera; marine mammals (whales, seals and dugong); introduced carnivores (Canidae and Felidae); introduced Leporidae (hares and rabbits); and introduced ungulates (Perissodactyla and Artiodactyla). In this chapter an attempt is made to compare and contrast the main features of the natural histories of these suites of species and, where appropriate, to comment on their resemblance to or difference from the metatherians. NATURAL HISTORY Ecology Diet. The native rodents are predominantly omnivorous. -

Ba3444 MAMMAL BOOKLET FINAL.Indd
Intot Obliv i The disappearing native mammals of northern Australia Compiled by James Fitzsimons Sarah Legge Barry Traill John Woinarski Into Oblivion? The disappearing native mammals of northern Australia 1 SUMMARY Since European settlement, the deepest loss of Australian biodiversity has been the spate of extinctions of endemic mammals. Historically, these losses occurred mostly in inland and in temperate parts of the country, and largely between 1890 and 1950. A new wave of extinctions is now threatening Australian mammals, this time in northern Australia. Many mammal species are in sharp decline across the north, even in extensive natural areas managed primarily for conservation. The main evidence of this decline comes consistently from two contrasting sources: robust scientifi c monitoring programs and more broad-scale Indigenous knowledge. The main drivers of the mammal decline in northern Australia include inappropriate fi re regimes (too much fi re) and predation by feral cats. Cane Toads are also implicated, particularly to the recent catastrophic decline of the Northern Quoll. Furthermore, some impacts are due to vegetation changes associated with the pastoral industry. Disease could also be a factor, but to date there is little evidence for or against it. Based on current trends, many native mammals will become extinct in northern Australia in the next 10-20 years, and even the largest and most iconic national parks in northern Australia will lose native mammal species. This problem needs to be solved. The fi rst step towards a solution is to recognise the problem, and this publication seeks to alert the Australian community and decision makers to this urgent issue. -

Australia's Biodiversity and Climate Change
Australia’s Biodiversity and Climate Change A strategic assessment of the vulnerability of Australia’s biodiversity to climate change A report to the Natural Resource Management Ministerial Council commissioned by the Australian Government. Prepared by the Biodiversity and Climate Change Expert Advisory Group: Will Steffen, Andrew A Burbidge, Lesley Hughes, Roger Kitching, David Lindenmayer, Warren Musgrave, Mark Stafford Smith and Patricia A Werner © Commonwealth of Australia 2009 ISBN 978-1-921298-67-7 Published in pre-publication form as a non-printable PDF at www.climatechange.gov.au by the Department of Climate Change. It will be published in hard copy by CSIRO publishing. For more information please email [email protected] This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from the Commonwealth. Requests and inquiries concerning reproduction and rights should be addressed to the: Commonwealth Copyright Administration Attorney-General's Department 3-5 National Circuit BARTON ACT 2600 Email: [email protected] Or online at: http://www.ag.gov.au Disclaimer The views and opinions expressed in this publication are those of the authors and do not necessarily reflect those of the Australian Government or the Minister for Climate Change and Water and the Minister for the Environment, Heritage and the Arts. Citation The book should be cited as: Steffen W, Burbidge AA, Hughes L, Kitching R, Lindenmayer D, Musgrave W, Stafford Smith M and Werner PA (2009) Australia’s biodiversity and climate change: a strategic assessment of the vulnerability of Australia’s biodiversity to climate change. -

Status Review, Disease Risk Analysis and Conservation Action Plan for The
Status Review, Disease Risk Analysis and Conservation Action Plan for the Bellinger River Snapping Turtle (Myuchelys georgesi) December, 2016 1 Workshop participants. Back row (l to r): Ricky Spencer, Bruce Chessman, Kristen Petrov, Caroline Lees, Gerald Kuchling, Jane Hall, Gerry McGilvray, Shane Ruming, Karrie Rose, Larry Vogelnest, Arthur Georges; Front row (l to r) Michael McFadden, Adam Skidmore, Sam Gilchrist, Bruno Ferronato, Richard Jakob-Hoff © Copyright 2017 CBSG IUCN encourages meetings, workshops and other fora for the consideration and analysis of issues related to conservation, and believes that reports of these meetings are most useful when broadly disseminated. The opinions and views expressed by the authors may not necessarily reflect the formal policies of IUCN, its Commissions, its Secretariat or its members. The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Jakob-Hoff, R. Lees C. M., McGilvray G, Ruming S, Chessman B, Gilchrist S, Rose K, Spencer R, Hall J (Eds) (2017). Status Review, Disease Risk Analysis and Conservation Action Plan for the Bellinger River Snapping Turtle. IUCN SSC Conservation Breeding Specialist Group: Apple Valley, MN. Cover photo: Juvenile Bellinger River Snapping Turtle © 2016 Brett Vercoe This report can be downloaded from the CBSG website: www.cbsg.org. 2 Executive Summary The Bellinger River Snapping Turtle (BRST) (Myuchelys georgesi) is a freshwater turtle endemic to a 60 km stretch of the Bellinger River, and possibly a portion of the nearby Kalang River in coastal north eastern New South Wales (NSW). -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica.