Non-Ketotic Hyperglycaemia and the Hemichorea- Hemiballismus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Upper Extremity Function in Persons with Tetraplegia: Relationships
Neurorehabilitation and Research Articles Neural Repair Volume 23 Number 5 June 2009 413-421 © 2009 The Author(s) 10.1177/1545968308331143 Upper Extremity Function in Persons with http://nnr.sagepub.com Tetraplegia: Relationships Between Strength, Capacity, and the Spinal Cord Independence Measure Claudia Rudhe, OT, MSc, and Hubertus J. A. van Hedel, PT, PhD Objective. To quantify the relationship between the Spinal Cord Independence Measure III (SCIM III), arm and hand muscle strength, and hand function tests in persons with tetraplegia. Methods. A total of 29 individuals with tetraplegia (motor level between cervical 4 and thoracic 1; sensory-motor complete and incomplete) participated. The total score, category scores, and separate items of the SCIM III were compared to the upper extremity motor score (UEMS), an extended manual muscle test (MMT) for 11 upper extremity muscles, and 6 functional capacity tests of the hand. Spearman’s correlation coefficients (rs) and regression analyses were performed. Results. The SCIM III sum score correlated well with the sum scores of the 3 tests (rs ≥ .76). The SCIM III self-care category correlated better with the tests (rs ≥ .80) compared to the other categories (rs ≤ .72). The SCIM III self-care item “grooming” highly correlated with muscle strength and hand capacity items (rs ≥ .80). A combination of hand muscle tests and the key grasping task explained over 90% of the variability in the self-care category scores. Conclusions. The SCIM III self-care category reflects upper extremity performance as it contains especially useful and valid items that relate to upper extremity function and capacity tests. -

Four Effective and Feasible Interventions for Hemi-Inattention
University of Puget Sound Sound Ideas School of Occupational Master's Capstone Projects Occupational Therapy, School of 5-2016 Four Effective and Feasible Interventions for Hemi- inattention Post CVA: Systematic Review and Collaboration for Knowledge Translation in an Inpatient Rehab Setting. Elizabeth Armbrust University of Puget Sound Domonique Herrin University of Puget Sound Christi Lewallen University of Puget Sound Karin Van Duzer University of Puget Sound Follow this and additional works at: http://soundideas.pugetsound.edu/ot_capstone Part of the Occupational Therapy Commons Recommended Citation Armbrust, Elizabeth; Herrin, Domonique; Lewallen, Christi; and Van Duzer, Karin, "Four Effective and Feasible Interventions for Hemi-inattention Post CVA: Systematic Review and Collaboration for Knowledge Translation in an Inpatient Rehab Setting." (2016). School of Occupational Master's Capstone Projects. 4. http://soundideas.pugetsound.edu/ot_capstone/4 This Article is brought to you for free and open access by the Occupational Therapy, School of at Sound Ideas. It has been accepted for inclusion in School of Occupational Master's Capstone Projects by an authorized administrator of Sound Ideas. For more information, please contact [email protected]. INTERVENTIONS FOR HEMI-INATTENTION IN INPATIENT REHAB Four Effective and Feasible Interventions for Hemi-inattention Post CVA: Systematic Review and Collaboration for Knowledge Translation in an Inpatient Rehab Setting. May 2016 This evidence project, submitted by Elizabeth Armbrust, -
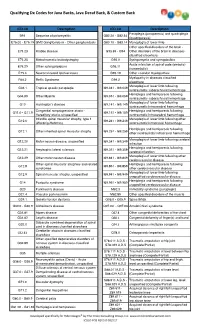
ICD-10 Backs
Qualifying Dx Codes for Java Backs, Java Decaf Back, & Custom Back ICD-10 Description ICD-10 Description Paraplegia (paraparesis) and quadriplegia B91 Sequelae of poliomyelitis G82.20 - G82.54 (quadriparesis) E75.00 - E75.19 GM2 Gangliosidosis - Other gangliosidosis G83.10 - G83.14 Monoplegia of lower limb Other specified disorders of the brain - E75.23 Krabbe disease G93.89 - G94 Other disorders of the brain in diseases classified elsewhere E75.25 Metachromatic leukodystrophy G95.0 Syringomyelia and syringobulbia Acute infarction of spinal code (embolic) E75.29 Other sphingolipidosis G95.11 (nonembolic) E75.4 Neuronal ceroid lipofuscinosis G95.19 Other vascular myelopathies Myelopathy in diseases classified F84.2 Rett's Syndrome G99.2 elsewhere Monoplegia of lower limb following G04.1 Tropical spastic paraplegia I69.041 - I69.049 nontraumatic subarachnoid hemorrhage Hemiplegia and hemiparesis following G04.89 Other Myelitis I69.051 - I69.059 nontraumatic subarachnoid hemorrhage Monoplegia of lower limb following G10 Huntington's disease I69.141 - I69.149 nontraumatic intracerebral hemorrhage Congenital nonprogressive ataxia - Hemiplegia and hemiparesis following G11.0 - G11.9 I69.151 - I69.159 Hereditary ataxia, unspecified nontraumatic intracerebral hemorrhage Infantile spinal muscular atrophy, type 1 Monoplegia of lower limb following other G12.0 I69.241 - I69.249 (Werdnig-Hoffman) nontraumatic intracranial hemorrhage Hemiplegia and hemiparesis following G12.1 Other inherited spinal muscular atrophy I69.251 - I69.259 other nontraumatic -

Traumatic Hemiparesis Associated with Type III Klippel-Feil Syndrome
online ML Comm www.jkns.or.kr J Korean Neurosurg Soc 42 : 145-148, 2007 Case Report Traumatic Hemiparesis Associated with Jin-Kyu Park, M.D. Type III Klippel-Feil Syndrome Han-Yong Huh, M.D. Kyeong-Sik Ryu, M.D. Chun-Kun Park, M.D. Klippel-Feil Syndrome (KFS) is a complex congenital syndrome of osseous and visceral anomalies. It is mainly associated with multi-level cervical spine fusion with hypermobile normal segments. Therefore, a patient with KFS can be at risk of severe neurological symptoms even after a minor trauma. We report a patient with type III KFS who developed a hemiparesis after a minor trauma and was successfully managed with operation. KEY WORDS : Hemiparesis·Klippel-Feil Syndrome·Trauma. INTRODUCTION Department of Neurosurgery In 1912, Klippel and Feil first described a syndrome that consisted of a clinical triad of short Kangnam St. Mary’s Hospital The Catholic University of Korea neck, limitation in head and neck movements and low posterior hairline. Currently, this Seoul, Korea syndrome called Klippel-Feil syndrome (KFS) is referred to a congenital fusion of two or more vertebrae7). Multi-level fused cervical vertebrae may become symptomatic during the rapid growth of adolescence or in adult life by the entrapment of the brain and/or the spinal cord. The classification of KFS is based on the site and extent of the cervical fusion9). Type I is applied to patients with an extensive cervical and upper thoracic spinal fusion. Type II refers to patients with one or two interspace fusions, often associated with hemivertebrae and occipitio- atlantal fusions. -

Reach and Palmar Grasp in Tetraplegics with Neuromuscular Electrical Stimulation
REACH AND PALMAR GRASP IN TETRAPLEGICS WITH NEUROMUSCULAR ELECTRICAL STIMULATION ALCANCE E PREENSÃO PALMAR EM TETRAPLÉGICOS COM ESTIMULAÇÃO ELÉTRICA NEUROMUSCULAR ORIGINAL ARTICLE ARTIGO ORIGINAL ALCANCE Y PRENSIÓN PALMAR EN TETRAPLÉJICOS CON ESTIMULACIÓN ELÉCTRICA NEUROMUSCULAR ARTÍCULO ORIGINAL Enio Walker Azevedo Cacho1 ABSTRACT (Physiotherapist) Roberta de Oliveira Cacho1 Objective: To evaluate the movement strategies of quadriplegics, assisted by neuromuscular electrical stimulation, (Physiotherapist) on reach and palmar grasp using objects of different weights. Methods: It was a prospective clinical trial. Four chronic Rodrigo Lício Ortolan2 quadriplegics (C5-C6), with injuries of traumatic origin, were recruited and all of them had their reach and palmar grasp (Electrical Engineer) movement captured by four infrared cameras and six retro-reflective markers attached to the trunk and right arm, as- 1 Núbia Maria Freire Vieira Lima sisted or not by neuromuscular electrical stimulation to the triceps, extensor carpi radialis longus, extensor digitorum (Physiotherapist) communis, flexor digitorum superficialis, opponens pollicis and lumbricals. It was measured by a Neurological and Edson Meneses da Silva Filho1 (Physiotherapist) Functional Classification of Spinal Cord Injuries of the American Spinal Injury Association, Functional Independence Alberto Cliquet Jr3,4 Measure and kinematic variables. Results: The patients were able to reach and execute palmar grasp in all cylinders (Electronics Engineer) using the stimulation sequences assisted by neuromuscular electrical stimulation. The quadriplegics produced lower peak velocity, a shorter time of movement and reduction in movement segmentation, when assisted by neuromuscular 1. Universidade Federal do Rio electrical stimulation. Conclusion: This study showed that reach and palmar grasp movement assisted by neuromuscular Grande do Norte, Faculdade de Ciências de Saúde do Trairi, electrical stimulation was able to produce motor patterns more similar to healthy subjects. -

Hemiballismus: /Etiology and Surgical Treatment by Russell Meyers, Donald B
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.13.2.115 on 1 May 1950. Downloaded from J. Neurol. Neurosurg. Psychiat., 1950, 13, 115. HEMIBALLISMUS: /ETIOLOGY AND SURGICAL TREATMENT BY RUSSELL MEYERS, DONALD B. SWEENEY, and JESS T. SCHWIDDE From the Division of Neurosurgery, State University of Iowa, College ofMedicine, Iowa City, Iowa Hemiballismus is a relatively uncommon hyper- 1949; Whittier). A few instances are on record in kinesia characterized by vigorous, extensive, and which the disorder has run an extended chronic rapidly executed, non-patterned, seemingly pur- course (Touche, 1901 ; Marcus and Sjogren, 1938), poseless movements involving one side of the body. while in one case reported by Lea-Plaza and Uiberall The movements are almost unceasing during the (1945) the abnormal movements are said to have waking state and, as with other hyperkinesias con- ceased spontaneously after seven weeks. Hemi- sidered to be of extrapyramidal origin, they cease ballismus has also been known to cease following during sleep. the supervention of a haemorrhagic ictus. Clinical Aspects Terminology.-There appears to be among writers on this subject no agreement regarding the precise Cases are on record (Whittier, 1947) in which the Protected by copyright. abnormal movements have been confined to a single features of the clinical phenomena to which the limb (" monoballismus ") or to both limbs of both term hemiballismus may properly be applied. sides (" biballismus ") (Martin and Alcock, 1934; Various authors have credited Kussmaul and Fischer von Santha, 1932). In a majority of recorded (1911) with introducing the term hemiballismus to instances, however, the face, neck, and trunk as well signify the flinging or flipping character of the limb as the limbs appear to have been involved. -

HCC Risk Adjustment Terminology: • HCC • CCV • AHA • RAF Score / Risk Score What Is Risk Adjustment?
HCC Risk Adjustment Terminology: • HCC • CCV • AHA • RAF Score / Risk Score What is Risk Adjustment? Who? What? Why? How? Redistributes Actuarial risk funds from plans Medicare To protect against based on with lower-risk Advantage Plans, adverse selection enrollees’ enrollees to plans Health Insurance and risk selection individual risk with higher-risk Exchanges, scores others enrollees RAF Calculation Demographic Health RAF Information StatusHealth Status Score Risk Score Higher risk scores represent members with a higher disease burden resulting in appropriately higher reimbursement. Lower risk scores represent a healthier population, but may also be due to: • Poor chart documentation • Poor diagnosis coding Why is this important? • Results in appropriate reimbursement • Augments the overall patient evaluation process • Allows us to stratify patients by risk • Metric for considering panel size, productivity, access, and coding education efforts Current Scope: Attributed Downside Plan Gain Share Lives Risk NGACO 24,876 80%/6.5 80%/6.5 91.5%- MCW 3,785 100% 100.65% Healthy 91.5%- 4,163 100% Saver 100.65% MA 16,561 50% TBD Current State: HCP-LAN APM Framework Category 3A: 83% Category 3B: 17% FFS APM HCC Risk Model • Diagnosis codes with RAF values are grouped into Condition Categories. • Diseases within a Condition Category are clinically related and are similar in respect to cost patterns. HCC Description Applicable Diagnoses to HCC Category RAF 111 Chronic Obstructive COPD Emphysema 0.346 Pulmonary Disease Chronic Bronchitis 108 Vascular Disease Atherosclerosis (of native or PVD 0.299 bypass grafts with or without Phlebitis and Thrombophlebitis complications [rest pain, (acute or chronic) claudication, etc]) Embolism of Veins (acute or Aortic Aneurysm chronic) (nonruptured) Diabetic Peripheral Angiopathy Arterial Aneurysm • There are 79 identified Condition Categories in 2018. -

Absence of Hemispatial Neglect Following Right Hemispherectomy in a 7-Year-Old Girl with Neonatal Stroke
Neurology Asia 2017; 22(2) : 149 – 154 CASE REPORTS Absence of hemispatial neglect following right hemispherectomy in a 7-year-old girl with neonatal stroke *1Jiqing Qiu PhD, *2Yu Cui MD, 3Lichao Sun MD, 1Bin QiPhD, 1Zhanpeng Zhu PhD *JQ Qiu and Y Cui contributed equally to this work Departments of 1Neurosurgery, 2Otolaryngology and 3Emergency Medicine, The First Hospital of Jilin University, Changchun, Jilin, China Abstract Neonatal stroke leads to cognitive deficits that may include hemispatial neglect. Hemispatial neglect is a syndrome after stroke that patients fail to be aware of stimuli on the side of space and body opposite a brain lesion. We report here a 7-year-old girl who suffered neonatal right brain stroke and underwent right hemispherectomy due to refractory epilepsy. Post-surgical observation of the child’s behavior and tests did not show any signs of hemispatial neglect. We concluded the spatial attention function of the child with neonatal stroke might be transferred to the contralateral side during early childhood. Key words: Epilepsy; hemispatial neglect; hemispherectomy; neonatal stroke; child. INTRODUCTION CASE REPORT Approximately one in every 4,000 newborns suffer The patient was a 7-year-old right-handed girl. from neonatal stroke, with a high mortalityrate.1-3 She was delivered with normal body weight. The survivors may develop varying degrees Gestation and delivery were uneventful. There of hemiparesis and low intelligence quotient was no family history of epilepsy. She had coma (IQ). Children with neonatal stroke, especially a few days after birth and was diagnosed with children with hemiplegia, have a high risk of neonatal stroke (Figure 1A-D). -

Functional Weakness and Sensory Disturbance J Stone, a Zeman, M Sharpe
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.73.3.241 on 1 September 2002. Downloaded from 241 PHYSICAL SIGNS Functional weakness and sensory disturbance J Stone, A Zeman, M Sharpe ............................................................................................................................. J Neurol Neurosurg Psychiatry 2002;73:241–245 In the diagnosis of functional weakness and sensory The history of the onset of the symptoms can disturbance, positive physical signs are as important as be particularly helpful. Patients with functional weakness will often describe symptoms sugges- absence of signs of disease. Motor signs, particularly tive of dissociation at the onset—either occurring Hoover’s sign, are more reliable than sensory signs, but in combination with panic, a physical trauma none should be used in isolation and must be (often minor), or spontaneously. In this context, “dissociation” refers to the weakening or loss of interpreted in the overall context of the presentation. It the normal sense of ownership of one’s actions should be borne in mind that a patient may have both a and sensations. Descriptions suggestive of disso- functional and an organic disorder. ciation include: “the leg felt as if it was not connected to me”, “I felt far away”, or “I was in a .......................................................................... place of my own”. ymptoms considered “functional,” “psycho- genic,” “medically unexplained,” or “hysteri- FUNCTIONAL WEAKNESS cal” account for up to one third of new refer- Preliminary observation S The physical assessment of functional weakness rals to neurology outpatient departments. Complaints of weakness or difficulty walking, should begin as the patient gets up from their often in combination with sensory disturbance, chair in the waiting room and end as they are represent a significant subgroup of these symp- leaving the consulting room (or the hospital). -

Evaluation of the Effect Oftreatment on Movement Disorders In
Journal ofNeurology, Neurosurgery, and Psychiatry 1993;56: 1113-1118 1113 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.56.10.1113 on 1 October 1993. Downloaded from Evaluation of the effect of treatment on movement disorders in astrocytomas of the basal ganglia and the thalamus Joachim K Krauss, Dieter F Braus, Mohsen Mohadjer, Fritz Nobbe, Fritz Mundinger Abstract Methods Twenty patients with movement disor- A total of 225 patients had astrocytomas ders associated with astrocytomas (grade grades I-IV of the basal ganglia or the thala- I-IV according to the WHO tumour clas- mus confirmed histologically by stereotactic sification) of the basal ganglia and the biopsy between 1965-86 in the department of thalamus were evaluated for the effects stereotaxy. Twenty were identified as having of treatment. Five patients had more an MD at the time of biopsy.5 The patients than one movement disorder when the were followed up and examined at various histological diagnosis was verified by times with recent follow ups in 1989, 90 or stereotactic biopsy. Twelve had tremors, 91. No follow up data concerning the tremor eight hemidystonia, three hemichorea, in patient 16 could be obtained. Some and one hemichorea/ballismus, and patients had serial film recordings over the myoclonus respectively. Ten patients years. Most of the long term surviving died during the follow up period, and for patients returned at regular intervals for con- the surviving patients foilow up periods trol CT scans. In addition, the relatives, ranged from 6-21 years. The movement attending physicians and neurologists were disorders changed over long periods of interviewed to compile the data. -

Cerebrovascular Accident (CVA) ICD-10-CM Clinical Overview
Cerebrovascular accident (CVA) ICD-10-CM Clinical overview Definition Some causes of hemorrhagic CVA A cerebrovascular accident, also known as a stroke, is an . Untreated or uncontrolled high blood pressure interruption or disruption of blood flow to the brain. Traumatic head and neck injuries When blood flow to an area of the brain stops, oxygen . Surgeries involving blood vessels of head and neck and nutrients cannot get to that area of the brain, and . Blood-thinning medications brain cells begin to die, resulting in permanent damage. Brain aneurysms (weak spots in walls of blood vessels in the brain) and other abnormalities of blood vessels Types in and around the brain . Ischemic: This type is usually caused by a blood clot . Bleeding disorders that blocks a blood vessel (artery) that supplies . Brain tumors oxygen-rich blood to the brain. Liver disease, which is associated with increased o Thrombotic stroke: A blood clot forms inside an bleeding in general artery that supplies blood to the brain, blocking blood flow. Risk factors o Embolic stroke: A blood clot forms in a vessel in . High blood pressure/hypertension another part of the body and then travels to and . Diabetes blocks a blood vessel in the brain. Obesity o Other types of ischemic stroke include very low . High cholesterol blood pressure or narrowing or tears in the lining . Cardiovascular disease of one of the blood vessels that carry blood to . Alcoholism the brain (i.e., carotid arteries), all of which . Smoking decrease blood flow to the brain. Age (older than 55) . Hemorrhagic: A blood vessel within the brain . -

Clinical Consequences of Stroke
EBRSR [Evidence-Based Review of Stroke Rehabilitation] 2 Clinical Consequences of Stroke Robert Teasell MD, Norhayati Hussein MBBS Last updated: March 2018 Abstract Cerebrovascular disorders represent the third leading cause of mortality and the second major cause of long-term disability in North America (Delaney and Potter 1993). The impairments associated with a stroke exhibit a wide diversity of clinical signs and symptoms. Disability, which is multifactorial in its determination, varies according to the degree of neurological recovery, the site of the lesion, the patient's premorbid status and the environmental support systems. Clinical evidence is reviewed as it pertains to stroke lesion location (cerebral, right & left hemispheres; lacunar and brain stem), related disorders (emotional, visual spatial perceptual, communication, fatigue, etc.) and artery(s) affected. 2. Clinical Consequences of Stroke pg. 1 of 29 www.ebrsr.com Table of Contents Abstract .............................................................................................................................................1 Table of Contents ...............................................................................................................................2 Introduction ......................................................................................................................................3 2.1 Localization of the Stroke ...........................................................................................................3 2.2 Cerebral