Ipsilateral Hemiparesis: the Forgotten History of This Paradoxical Neurological Sign
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dissemination of Brain Inflammation in Traumatic Brain Injury
Cellular & Molecular Immunology www.nature.com/cmi REVIEW ARTICLE Dissemination of brain inflammation in traumatic brain injury Kaibin Shi1,2,3, Jianning Zhang1, Jing-fei Dong4 and Fu-Dong Shi1,2,3 Traumatic brain injury (TBI) is recognized as a global health problem due to its increasing occurrence, challenging treatment, and persistent impacts on brain pathophysiology. Neural cell death in patients with TBI swiftly causes inflammation in the injured brain areas, which is recognized as focal brain inflammation. Focal brain inflammation causes secondary brain injury by exacerbating brain edema and neuronal death, while also exerting divergent beneficial effects, such as sealing the damaged limitans and removing cellular debris. Recent evidence from patients with TBI and studies on animal models suggest that brain inflammation after TBI is not only restricted to the focal lesion but also disseminates to remote areas of the brain. The dissemination of inflammation has been detected within days after the primary injury and persists chronically. This state of inflammation may be related to remote complications of TBI in patients, such as hyperthermia and hypopituitarism, and may lead to progressive neurodegeneration, such as chronic traumatic encephalopathy. Future studies should focus on understanding the mechanisms that govern the initiation and propagation of brain inflammation after TBI and its impacts on post-trauma brain pathology. Keywords: disseminated brain inflammation; traumatic brain injury; microglia; post-injury neurodegeneration Cellular & Molecular Immunology (2019) 16:523–530; https://doi.org/10.1038/s41423-019-0213-5 INTRODUCTION the first responders to the injury, followed by activated astrocytes Traumatic brain injury (TBI) encompasses mechanical brain injuries and endothelial cells. -

Prognostic Implications of Total Hemispheric Glucose Metabolism
Journal of Nuclear Medicine, published on October 27, 2016 as doi:10.2967/jnumed.116.180398 Prognostic Implications of Total Hemispheric Glucose Metabolism Ratio in Cerebro-Cerebellar Diaschisis *Eivind Antonsen Segtnan1,2, Peter Grupe1, Jens Ole Jarden3, Oke Gerke1,4,Jana Ivanidze5 Sofie Bæk Christlieb1, Caius Constantinescu1, John Erling Pedersen1, Sina Houshmand6, Søren Hess1,7,8 Mojtaba Zarei9, Albert Gjedde2,10, Abass Alavi6, Poul F. Høilund-Carlsen1,8 1Department of Nuclear Medicine, Odense University Hospital, Odense, Denmark 2 University of Southern Denmark, Odense, Denmark 3Department of Neurology, Herlev University Hospital, Copenhagen, Denmark 4Centre of Health Economics Research, Odense, University of Southern Denmark, Denmark 5Department of Diagnostic Radiology, Weill Cornell Medical College, NewYork-Presbyterian Hospital, New York, NY, United States 6Division of Nuclear Medicine, Department of Radiology, Perelman School of Medicine, Hospital of the University of Pennsylvania, Philadelphia, PA, USA 7Department of Radiology and Nuclear Medicine, Hospital of Southwest Jutland, Esbjerg, Denmark 8Department of Clinical Research, Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark 9National Brain Mapping Centre, Shahid Beheshti University (Medical and General Campus), Tehran, Iran. 10Department of Neuroscience and Pharmacology, Panum Institute, University of Copenhagen, Copenhagen, Denmark 1 *Corresponding author Eivind Antonsen Segtnan ( Medicin student) , Allegade 34, 2.tv, 5000 Odense C, Denmark, Tel: +45 3044 8498, e-mail: [email protected] 2 Abstract Purpose: Diaschisis denotes brain dysfunction remote from a focal brain lesion. We have quantified diaschisis and investigated its prognostic value in glioma. Methods and material: We compared 50 18F-FDG-PET-CT studies collected prospectively from 14 patients with supratentorial glioma (5 men and 9 women aged 35-77 years) with 10 single scans from healthy controls aged 43-75 years. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Changes Caused by Stroke
Recovery Frontal lobe Parietal lobe let’s talk about controls personality, controls speech and reasoning, parts of sensation (touch and Changes speech, and muscles pressure) Caused by Stroke Your brain controls how you move, feel, communicate, think and act. Brain injury from a stroke may affect any of these abilities. Some changes are common no matter which side of the brain the injury is on. Others Temporal lobe are based on which side of the brain Occipital lobe controls hearing, the stroke injures. speech, and short- controls vision term memory What are the most common general What are common changes with a effects of stroke? right-brain injury? • Hemiparesis (weakness on one side of the body) or • Paralysis or weakness on the left side of the body. hemiplegia (paralysis on one side of the body) • One-sided neglect, which is a lack of awareness of the • Dysarthria (difficulty speaking or slurred speech), or left side of the body. It may also be a lack of awareness dysphagia (trouble swallowing) of what is going on to the survivor’s left. For example, • Fatigue they may only eat from the right side of their plate, ignoring the left side of the plate. • Loss of emotional control and changes in mood • Behavior may be more impulsive and less cautious • Cognitive changes (problems with memory, judgment, than before. problem-solving or a combination of these) • It may be harder for the survivor to understand facial • Behavior changes (personality changes, improper expressions and tone of voice. They also may have less language or actions) expression in their own face and tone of voice when • Decreased field of vision (inability to see peripheral communicating. -

Myelopathy—Paresis and Paralysis in Cats
Myelopathy—Paresis and Paralysis in Cats (Disorder of the Spinal Cord Leading to Weakness and Paralysis in Cats) Basics OVERVIEW • “Myelopathy”—any disorder or disease affecting the spinal cord; a myelopathy can cause weakness or partial paralysis (known as “paresis”) or complete loss of voluntary movements (known as “paralysis”) • Paresis or paralysis may affect all four limbs (known as “tetraparesis” or “tetraplegia,” respectively), may affect only the rear legs (known as “paraparesis” or “paraplegia,” respectively), the front and rear leg on the same side (known as “hemiparesis” or “hemiplegia,” respectively) or only one limb (known as “monoparesis” or “monoplegia,” respectively) • Paresis and paralysis also can be caused by disorders of the nerves and/or muscles to the legs (known as “peripheral neuromuscular disorders”) • The spine is composed of multiple bones with disks (intervertebral disks) located in between adjacent bones (vertebrae); the disks act as shock absorbers and allow movement of the spine; the vertebrae are named according to their location—cervical vertebrae are located in the neck and are numbered as cervical vertebrae one through seven or C1–C7; thoracic vertebrae are located from the area of the shoulders to the end of the ribs and are numbered as thoracic vertebrae one through thirteen or T1–T13; lumbar vertebrae start at the end of the ribs and continue to the pelvis and are numbered as lumbar vertebrae one through seven or L1–L7; the remaining vertebrae are the sacral and coccygeal (tail) vertebrae • The brain -

Non-Ketotic Hyperglycaemia and the Hemichorea- Hemiballismus
This open-access article is distributed under ARTICLE Creative Commons licence CC-BY-NC 4.0. Non-ketotic hyperglycaemia and the hemichorea- hemiballismus syndrome – a rare paediatric presentation M P K Hauptfleisch, MB BCh, MMed Paeds, FCPaed (SA), Cert Paed Neuro (SA); J L Rodda, MB BCh, FCPaed (SA) Department of Paediatrics, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa and Chris Hani Baragwanath Academic Hospital, Johannesburg, Africa Corresponding author: M P K Hauptfleisch ([email protected]) Hemichorea-hemiballismus may be due to non-ketotic hyperglycaemia, but this condition has rarely been described in paediatrics. We describe the case of a 13-year-old girl with newly diagnosed type 1 diabetes and acute onset of left-sided choreoathetoid movements. Neuroimaging revealed an area of hyperintensity in the right basal ganglia. Her blood glucose level at the time was 19 mmol/L, and there was no ketonuria. The hemiballismus improved with risperidone and glycaemic control. Repeat neuroimaging 4 months later showed complete resolution of the hyperintensities seen. S Afr J Child Health 2018;12(4):134-136. DOI:10.7196/SAJCH.2018.v12i4.1535 Non-ketotic hyperglycaemic hemichorea-hemiballismus (NKHHC) is a rare, reversible condition, with the clinical and radiological signs usually resolving within 6 months, following correction of hyperglycaemia.[1] The condition has previously been described as specifically affecting the elderly, with very few cases described in children and adolescents.[2] We describe a paediatric patient presenting with the classic clinical and radiological findings of NKHHC. Case A 13-year-old female presented to hospital with severe cramps in her left hand. -

Associations of Pathological Diagnosis and Genetic Abnormalities In
www.nature.com/scientificreports OPEN Associations of pathological diagnosis and genetic abnormalities in meningiomas with the embryological origins of the meninges Atsushi Okano1, Satoru Miyawaki1*, Hiroki Hongo1, Shogo Dofuku1, Yu Teranishi1, Jun Mitsui2, Michihiro Tanaka3, Masahiro Shin1, Hirofumi Nakatomi1 & Nobuhito Saito1 Certain driver mutations and pathological diagnoses are associated with the anatomical site of meningioma, based on which the meninges have diferent embryological origins. We hypothesized that mutations and pathological diagnoses of meningiomas are associated with diferent embryological origins. We comprehensively evaluated associations among tumor location, pathological diagnosis (histological type), and genetic alterations including AKT1, KLF4, SMO, POLR2A, and NF2 mutations and 22q deletion in 269 meningioma cases. Based on the embryological origin of meninges, the tumor locations were as follows: neural crest, paraxial mesodermal, and dorsal mesodermal origins. Tumors originating from the dura of certain embryologic origin displayed a signifcantly diferent pathological diagnoses and genetic abnormality ratio. For instance, driver genetic mutations with AKT1, KLF4, SMO, and POLR2A, were signifcantly associated with the paraxial mesodermal origin (p = 1.7 × 10−10). However, meningiomas with NF2-associated mutations were signifcantly associated with neural crest origin (p = 3.9 × 10–12). On analysis of recurrence, no diference was observed in embryological origin. However, POLR2A mutation was a risk factor for the tumor recurrence (p = 1.7 × 10−2, Hazard Ratio 4.08, 95% Confdence Interval 1.28–13.0). Assessment of the embryological origin of the meninges may provide novel insights into the pathomechanism of meningiomas. Meningiomas are the most common primary intracranial tumors accounting for 20% of all such tumors. -

Upper Extremity Function in Persons with Tetraplegia: Relationships
Neurorehabilitation and Research Articles Neural Repair Volume 23 Number 5 June 2009 413-421 © 2009 The Author(s) 10.1177/1545968308331143 Upper Extremity Function in Persons with http://nnr.sagepub.com Tetraplegia: Relationships Between Strength, Capacity, and the Spinal Cord Independence Measure Claudia Rudhe, OT, MSc, and Hubertus J. A. van Hedel, PT, PhD Objective. To quantify the relationship between the Spinal Cord Independence Measure III (SCIM III), arm and hand muscle strength, and hand function tests in persons with tetraplegia. Methods. A total of 29 individuals with tetraplegia (motor level between cervical 4 and thoracic 1; sensory-motor complete and incomplete) participated. The total score, category scores, and separate items of the SCIM III were compared to the upper extremity motor score (UEMS), an extended manual muscle test (MMT) for 11 upper extremity muscles, and 6 functional capacity tests of the hand. Spearman’s correlation coefficients (rs) and regression analyses were performed. Results. The SCIM III sum score correlated well with the sum scores of the 3 tests (rs ≥ .76). The SCIM III self-care category correlated better with the tests (rs ≥ .80) compared to the other categories (rs ≤ .72). The SCIM III self-care item “grooming” highly correlated with muscle strength and hand capacity items (rs ≥ .80). A combination of hand muscle tests and the key grasping task explained over 90% of the variability in the self-care category scores. Conclusions. The SCIM III self-care category reflects upper extremity performance as it contains especially useful and valid items that relate to upper extremity function and capacity tests. -

Four Effective and Feasible Interventions for Hemi-Inattention
University of Puget Sound Sound Ideas School of Occupational Master's Capstone Projects Occupational Therapy, School of 5-2016 Four Effective and Feasible Interventions for Hemi- inattention Post CVA: Systematic Review and Collaboration for Knowledge Translation in an Inpatient Rehab Setting. Elizabeth Armbrust University of Puget Sound Domonique Herrin University of Puget Sound Christi Lewallen University of Puget Sound Karin Van Duzer University of Puget Sound Follow this and additional works at: http://soundideas.pugetsound.edu/ot_capstone Part of the Occupational Therapy Commons Recommended Citation Armbrust, Elizabeth; Herrin, Domonique; Lewallen, Christi; and Van Duzer, Karin, "Four Effective and Feasible Interventions for Hemi-inattention Post CVA: Systematic Review and Collaboration for Knowledge Translation in an Inpatient Rehab Setting." (2016). School of Occupational Master's Capstone Projects. 4. http://soundideas.pugetsound.edu/ot_capstone/4 This Article is brought to you for free and open access by the Occupational Therapy, School of at Sound Ideas. It has been accepted for inclusion in School of Occupational Master's Capstone Projects by an authorized administrator of Sound Ideas. For more information, please contact [email protected]. INTERVENTIONS FOR HEMI-INATTENTION IN INPATIENT REHAB Four Effective and Feasible Interventions for Hemi-inattention Post CVA: Systematic Review and Collaboration for Knowledge Translation in an Inpatient Rehab Setting. May 2016 This evidence project, submitted by Elizabeth Armbrust, -

Clinical Decision Making in Neurology
CLINICAL DECISION MAKING IN NEUROLOGY The Neurological Examination The goals of the neurological examination are to detect the presence of a neurologic disease and to determine the location of the lesion within the nervous system. The neurologic examination should be performed in a systematic manner in order to assure that the animal's neurologic status is completely evaluated. The parts of a customary neurologic examination include evaluation of the head (mentation and cranial nerves), gait, limbs (postural reactions and spinal reflexes) and pain/nociception (normal and abnormal pain responses). Gait Evaluation I find gait evaluation the most useful and important part of the neurologic exam. Clinical evaluation of gait usually involves observation of the animal's movements while walking. This is best accomplished by having a handler walk the animal over a flat, non-slippery area. I typically evaluate gait by having a handler walk the animal approximately 100 feet, away and back, as well as circling clockwise and counterclockwise. I have the animal short on the leash and under good control and have the dog typically walk slowly. I may have them walk on and off the curb. Overall, evaluation is made by watching for paresis, ataxia, stride length, lameness, stiffness, spasticity, dysmetria and shuffling or scuffing of limbs or digits. I also note position of head and tail during gait evaluation. Gait analysis is broken down between the axial and appendicular skeleton. The axial vertebral column is made up of many joints and is divided into anatomical segments. The axial portion includes the head, neck, thoracic, lumbar, lumbar/pelvic and tail. -
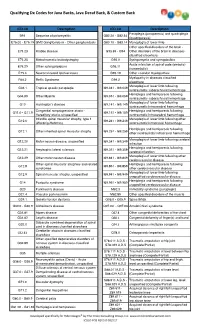
ICD-10 Backs
Qualifying Dx Codes for Java Backs, Java Decaf Back, & Custom Back ICD-10 Description ICD-10 Description Paraplegia (paraparesis) and quadriplegia B91 Sequelae of poliomyelitis G82.20 - G82.54 (quadriparesis) E75.00 - E75.19 GM2 Gangliosidosis - Other gangliosidosis G83.10 - G83.14 Monoplegia of lower limb Other specified disorders of the brain - E75.23 Krabbe disease G93.89 - G94 Other disorders of the brain in diseases classified elsewhere E75.25 Metachromatic leukodystrophy G95.0 Syringomyelia and syringobulbia Acute infarction of spinal code (embolic) E75.29 Other sphingolipidosis G95.11 (nonembolic) E75.4 Neuronal ceroid lipofuscinosis G95.19 Other vascular myelopathies Myelopathy in diseases classified F84.2 Rett's Syndrome G99.2 elsewhere Monoplegia of lower limb following G04.1 Tropical spastic paraplegia I69.041 - I69.049 nontraumatic subarachnoid hemorrhage Hemiplegia and hemiparesis following G04.89 Other Myelitis I69.051 - I69.059 nontraumatic subarachnoid hemorrhage Monoplegia of lower limb following G10 Huntington's disease I69.141 - I69.149 nontraumatic intracerebral hemorrhage Congenital nonprogressive ataxia - Hemiplegia and hemiparesis following G11.0 - G11.9 I69.151 - I69.159 Hereditary ataxia, unspecified nontraumatic intracerebral hemorrhage Infantile spinal muscular atrophy, type 1 Monoplegia of lower limb following other G12.0 I69.241 - I69.249 (Werdnig-Hoffman) nontraumatic intracranial hemorrhage Hemiplegia and hemiparesis following G12.1 Other inherited spinal muscular atrophy I69.251 - I69.259 other nontraumatic -

Mitochondrial Physiology Within Myelinated Axons in Health and Disease : an Energetic Interplay Between Counterparts Gerben Van Hameren
Mitochondrial physiology within myelinated axons in health and disease : an energetic interplay between counterparts Gerben Van Hameren To cite this version: Gerben Van Hameren. Mitochondrial physiology within myelinated axons in health and disease : an energetic interplay between counterparts. Human health and pathology. Université Montpellier, 2018. English. NNT : 2018MONTT084. tel-02053421 HAL Id: tel-02053421 https://tel.archives-ouvertes.fr/tel-02053421 Submitted on 1 Mar 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE POUR OBTENIR LE GRADE DE DOCTEUR DE L’UNIVERSITÉ DE M ONTPELLIER En Biologie Santé École doctorale CBS2 Institut des Neurosciences de Montpellier MITOCHONDRIAL PHYSIOLOGY WITHIN MYELINATED AXONS IN HEALTH AND DISEASE AN ENERGETIC INTERPLAY BETWEEN COUNTERPARTS Présentée par Gerben van Hameren Le 23 Novembre 2018 Sous la direction de Dr. Nicolas Tricaud Devant le jury composé de Prof. Pascale Belenguer, Centre de Recherches sur la Cognition Animale Toulouse Professeur d’université Dr. Guy Lenaers, Mitochondrial Medicine Research Centre Angers Directeur de recherche Dr. Don Mahad, University of Edinburgh Senior clinical lecturer Invité Dr. Marie-Luce Vignais, Institute for Regenerative Medicine & Biotherapy, Montpellier Chargé de recherche 0 Table of contents Prologue .................................................................................................................................................