Mitochondrial Physiology Within Myelinated Axons in Health and Disease : an Energetic Interplay Between Counterparts Gerben Van Hameren
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
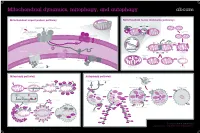
Mitochondrial Dynamics, Mitophagy, and Autophagy
Mitochondrial dynamics, mitophagy, and autophagy Mitochondrial import protein pathway Mitochondrial fusion and fission pathways iΔΨm, (OXPHOSh) Ca2+ Fusion ++++ (Cell differentiationi) N Internal signal β-barrel outer Opa1 SH ER sequence SH membrane precursors Mfn1/2 SH SH GTP GTP C Inner membrane and GTP GTP Outer membrane Matrix carrier precursors 22 70 GTP fusion (N-terminal presequences) 35 GTP 20 37 Tom complex 5 Sam complex Opa1 Cytosol Outer 6 Sam50 7 Mdm10 membrane DRP1 Fis1 Inner membrane fusion Tim8-Tim13 Mim 1 Tom40 Bax/Bak apoptosis Outer membrane HR2 regions S-S Tim9-Tim10 S-S Inner Erv1 95 A 54 mitochondrial S-S Reorganization Mia40 space 21 Fission sequestration 50 18 Tim22 Translocation Depolarization to meet iΔΨm ATP needs Tim23 KREBS Cycle NADH FADH2 17 Oxa1 Tim 22 complex Mia Mba1 Mdm I ATP 44 38 H+ II 17 H+ Inner membrane 16 +++ III ADP - Pi 18 Ribosome Oxa H+ IV mtHsp70 H+ ATP +++ Mge1 MPP Healthy mitochondrion Mitophagy Matrix Tim 23 complex Mitophagy pathway Autophagy pathway Lysosomal hydroiase Lysosome Hypoxia, Nutrient/Growth Rapamycin Starvation condition Ubiquitin Cytosol Parkin factor deprivation Lamp Stress Damaged mitochondrion Parkin Parkin iΔΨm Parkin MARF PINK1 PINK1 PINK1 Mfn1 VDAC1 Parkin AMPK mTOR Mfn2 PINK1 PINK1 Phagophore Autophagosome Autophagolyosome PARL LC3-II PINK1-L PINK1-S Parkin P FIS1 ATG13 ULK LC3-II Membrane PINK1 PINK1 FIP200 PINK1 MIRO2 P Bak PINK1 MIRO1 Parkin Parkin Parkin Cytoplasmic Cytosol proLC3 macromolecule LC3-II Atg4B Atg7/LC3-I Atg5/Atg12/Atg16 Organelle Atg9 PE Atg18 Atg2 -

Dissemination of Brain Inflammation in Traumatic Brain Injury
Cellular & Molecular Immunology www.nature.com/cmi REVIEW ARTICLE Dissemination of brain inflammation in traumatic brain injury Kaibin Shi1,2,3, Jianning Zhang1, Jing-fei Dong4 and Fu-Dong Shi1,2,3 Traumatic brain injury (TBI) is recognized as a global health problem due to its increasing occurrence, challenging treatment, and persistent impacts on brain pathophysiology. Neural cell death in patients with TBI swiftly causes inflammation in the injured brain areas, which is recognized as focal brain inflammation. Focal brain inflammation causes secondary brain injury by exacerbating brain edema and neuronal death, while also exerting divergent beneficial effects, such as sealing the damaged limitans and removing cellular debris. Recent evidence from patients with TBI and studies on animal models suggest that brain inflammation after TBI is not only restricted to the focal lesion but also disseminates to remote areas of the brain. The dissemination of inflammation has been detected within days after the primary injury and persists chronically. This state of inflammation may be related to remote complications of TBI in patients, such as hyperthermia and hypopituitarism, and may lead to progressive neurodegeneration, such as chronic traumatic encephalopathy. Future studies should focus on understanding the mechanisms that govern the initiation and propagation of brain inflammation after TBI and its impacts on post-trauma brain pathology. Keywords: disseminated brain inflammation; traumatic brain injury; microglia; post-injury neurodegeneration Cellular & Molecular Immunology (2019) 16:523–530; https://doi.org/10.1038/s41423-019-0213-5 INTRODUCTION the first responders to the injury, followed by activated astrocytes Traumatic brain injury (TBI) encompasses mechanical brain injuries and endothelial cells. -

Involvement of Impaired Autophagy and Mitophagy in Neuro-2A Cell
www.nature.com/scientificreports OPEN Involvement of impaired autophagy and mitophagy in Neuro-2a cell damage under hypoxic and/or high- Received: 19 May 2017 Accepted: 15 January 2018 glucose conditions Published: xx xx xxxx Yufei Song, Yu Du, Wenying Zou, Yan Luo, Xiaojie Zhang & Jianliang Fu Chronic cerebral hypoperfusion (CCH) plays an insidious role in the development of cognitive impairment. Considerable evidence suggests that Diabetes Mellitus (DM) as a vascular risk factor may exacerbate CCH and is closely related to cognitive decline. Dysregulation of autophagy is known to be associated with the pathogenesis of neurodegenerative diseases such as Alzheimer’s disease. To elucidate the role of autophagy in CCH- and/or DM-related pathogenesis, mouse neuroblastoma Neuro-2a cells were exposed to hypoxia and/or high glucose for 48 h, mimicking CCH complicated with DM pathologies. Chronic hypoxia reduced cell proliferation and increased levels of cleaved caspase-3, whereas high glucose had no obvious synergistic toxic efect. Accumulation of autophagic vacuoles under hypoxia may be due to both autophagy impairment and induction, with the former accounting for Neuro-2a cell death. Additionally, aberrant accumulation of mitochondria in Neuro-2a cells may be attributed to insufcient BNIP3-mediated mitophagy due to poor interaction between BNIP3 and LC3-II. Despite the lack of a signifcant cytotoxic efect of high glucose under our experimental conditions, our data indicated for the frst time that impaired autophagy degradation and inefcient BNIP3-mediated mitophagy may constitute mechanisms underlying neuronal cell damage during chronic hypoxia. Chronic cerebral hypoperfusion (CCH) is a normal process related to ageing that likely contributes to age-related memory loss1. -

Calcium Ion Distribution in Nascent Pioneer Axons and Coupled Preaxonogenesis Neurons in Situ
The Journal of Neuroscience, May 1991, 1 I(5): 1300-l 308 Calcium Ion Distribution in Nascent Pioneer Axons and Coupled Preaxonogenesis Neurons in situ David Bentley,’ Peter B. Guthrie,2 and Stanley B. Kater2 ‘Department of Molecular and Cell Biology, University of California, Berkeley, California 94720 and 2Department of Anatomy and Neurobiology, Colorado State University, Fort Collins, Colorado 80523 The “calcium hypothesis” of regulation of growth cone mo- and Lux, 1989). In a variety of cell types, growth cone motility tility and neurite elongation has derived from analysis of a and neurite elongation have been correlated with intracellular variety of neurons growing in vitro. It proposes that calcium calcium concentration (Anglister et al., 1982; Connor, 1986; ion concentration within growth cones is an important reg- Cohan et al., 1987; Goldberg, 1988; Lankford and Letoumeau, ulator of motility and growth. We now extend this analysis 1989; Silver et al., 1989, 1990; Tolkovsky et al., 1990). Inter- by investigating calcium concentrations within growth cones actions with both soluble and substratemolecules influence in- and nascent neurites of identified embryonic neurons grow- tracellular calcium levels. Exposure of growth conesto a variety ing on their normal substrate in situ. of neurotransmitters can arrest or otherwise regulate growth The pair of Til pioneer neurons are the first to extend (Haydon et al., 1984; Connor et al., 1987; Mattson et al., 1988; axons in limb buds of grasshopper embryos. Their growth McCobb and Kater, 1988; McCobb et al., 1988). This effect cones migrate along a stereotyped pathway, where they en- appearsto be mediated, at least in part, by alteration of intra- counter a series of guidance cues, including preaxonoge- cellular calcium levels through voltage-gated calcium channels nesis afferent neurons (guidepost cells). -

Mitoq Inhibits Hepatic Stellate Cell Activation and Liver Fibrosis by Enhancing PINK1/Parkin-Mediated Mitophagy
MitoQ Inhibits Hepatic Stellate Cell Activation and Liver Fibrosis by Enhancing PINK1/parkin-mediated Mitophagy Shi-Ying Dou Second Hospital of Hebei Medical University Jiu-Na Zhang Second Hospital of Hebei Medical University Xiao-Li Xie Second Hospital of Hebei Medical University Ting Liu Second Hospital of Hebei Medical University Jun-Li Hu Second Hospital of Hebei Medical University Xiao-Yu Jiang Second Hospital of Hebei Medical University Miao-Miao Wang Second Hospital of Hebei Medical University Hui-Qing Jiang ( [email protected] ) Second Hospital of Hebei Medical University https://orcid.org/0000-0002-5235-1457 Research Article Keywords: Liver brosis, hepatic stellate cell, ubiquinone, PINK1 mitophagy Posted Date: March 30th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-344963/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/18 Abstract Mitophagy plays an important role in the activation of hepatic stellate cells (HSCs). Mitochondria- targeted ubiquinone (MitoQ) is a mitochondria-targeted antioxidant that reduces the production of intracellular reactive oxygen species (ROS). However, its relationship with mitophagy remains unclear. This study evaluated mitophagy during HSC activation and the effects of MitoQ on mitophagy in cell culture and in an animal model of the activation of HSCs. We found that MitoQ reduced the activation of HSCs and alleviated hepatic brosis. While activation of primary HSCs or LX-2 cells was associated with reduced PINK1/parkin-mediated mitophagy, MitoQ reduced intracellular ROS levels, enhanced PINK1/parkin-mediated mitophagy, and inhibited the activation of HSCs. After knocking down the key mitophagy-related protein, PINK1, in LX-2 cells to block mitophagy, MitoQ intervention failed to inhibit HSC activation. -

Robo1 and Robo2 Control the Development of the Lateral Olfactory Tract
The Journal of Neuroscience, March 14, 2007 • 27(11):3037–3045 • 3037 Development/Plasticity/Repair Robo1 and Robo2 Control the Development of the Lateral Olfactory Tract Coralie Fouquet,1,2 Thomas Di Meglio,1,2 Le Ma,4 Takahiko Kawasaki,3 Hua Long,4 Tatsumi Hirata,3 Marc Tessier-Lavigne,3 Alain Che´dotal,1,2 and Kim T. Nguyen-Ba-Charvet1,2 1Centre National de la Recherche Scientifique and 2Universite´ Pierre et Marie Curie-Paris 6, Unite´ Mixte de Recherche 7102, Paris, 75005 France, 3Division of Brain Function, National Institute of Genetics, Graduate University for advanced Studies (Sokendai), Yata 1111, Mishima 411-8540, Japan, and 4Howard Hughes Medical Institute, Department of Biological sciences, Stanford University, Stanford, California 94305 The development of olfactory bulb projections that form the lateral olfactory tract (LOT) is still poorly understood. It is known that the septum secretes Slit1 and Slit2 which repel olfactory axons in vitro and that in Slit1Ϫ/Ϫ;Slit2Ϫ/Ϫ mutant mice, the LOT is profoundly disrupted.However,theinvolvementofSlitreceptors,theroundabout(Robo)proteins,inguidingLOTaxonshasnotbeendemonstrated. We show here that both Robo1 and Robo2 receptors are expressed on early developing LOT axons, but that only Robo2 is present at later developmental stages. Olfactory bulb axons from Robo1Ϫ/Ϫ;Robo2Ϫ/Ϫ double-mutant mice are not repelled by Slit in vitro. The LOT develops normally in Robo1Ϫ/Ϫ mice, but is completely disorganized in Robo2Ϫ/Ϫ and Robo1Ϫ/Ϫ;Robo2Ϫ/Ϫ double-mutant embryos, with many LOT axons spreading along the ventral surface of the telencephalon. Finally, the position of lot1-expressing cells, which have been proposed to be the LOT guidepost cells, appears unaffected in Slit1Ϫ/Ϫ;Slit2Ϫ/Ϫ mice and in Robo1Ϫ/Ϫ;Robo2Ϫ/Ϫ mice. -

Gp78 E3 Ubiquitin Ligase Mediates Both Basal and Damage-Induced Mitophagy
bioRxiv preprint doi: https://doi.org/10.1101/407593; this version posted September 3, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Gp78 E3 ubiquitin ligase mediates both basal and damage-induced mitophagy Bharat Joshi, Yahya Mohammadzadeh, Guang Gao and Ivan R. Nabi Department of Cellular and Physiological Sciences, Life Sciences Institute, University of British Columbia, Vancouver, BC V6T 1Z3, Canada #Running title: Gp78 control of mitophagy §To whom correspondence should be addressed: Ivan R. Nabi, Department of Cellular and Physiological Sciences, Life Sciences Institute, University of British Columbia, 2350 Health Sciences Mall, Vancouver, BC V6T 1Z3 Canada. Tel: +1-(604) 822-7000 E-mail: [email protected] Key words: Gp78 ubiquitin ligase; mitochondria; autophagy; PINK1; Parkin bioRxiv preprint doi: https://doi.org/10.1101/407593; this version posted September 3, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Mitophagy, the elimination of mitochondria by the autophagy machinery, evolved to monitor mitochondrial health and maintain mitochondrial integrity. PINK1 is a sensor of mitochondrial health that recruits Parkin and other mitophagy-inducing ubiquitin ligases to depolarized mitochondria. However, mechanisms underlying mitophagic control of mitochondrial homeostasis, basal mitophagy, remain poorly understood. The Gp78 E3 ubiquitin ligase, an endoplasmic reticulum membrane protein, induces mitochondrial fission, endoplasmic reticulum- mitochondria contacts and mitophagy of depolarized mitochondria. CRISPR/Cas9 knockout of Gp78 in HT-1080 fibrosarcoma cells results in reduced ER-mitochondria contacts, increased mitochondrial volume and resistance to CCCP-induced mitophagy. -

Technische Universität München
TECHNISCHE UNIVERSITÄT MÜNCHEN Lehrstuhl für Entwicklungsgenetik Molecular mechanisms that govern the establishment of sensory-motor networks Rosa-Eva Hüttl Vollständiger Abdruck der von der Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt der Technischen Universität München zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigten Dissertation. Vorsitzender: Univ.-Prof. Dr. E. Grill Prüfer der Dissertation: 1. Univ.-Prof. Dr. W. Wurst 2. Univ.-Prof. Dr. H. Luksch Die Dissertation wurde am 08.12.2011 bei der Technischen Universität München eingereicht und durch die Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt am 27.02.2012 angenommen. Erklärung Hiermit erkläre ich an Eides statt, dass ich die der Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt der Technischen Universität München zur Promotionsprüfung vorgelegte Arbeit mit dem Titel „Molecular mechanisms that govern the establishment of sensory-motor networks“ am Lehrstuhl für Entwicklungsgenetik unter der Anleitung und Betreuung durch Univ.-Prof. Dr. Wolfgang Wurst ohne sonstige Hilfe erstellt und bei der Abfassung nur die gemäß § 6 Abs. 5 angegebenen Hilfsmittel benutzt habe. Ich habe keine Organisation eingeschaltet, die gegen Entgelt Betreuerinnen und Betreuer für die Anfertigung von Dissertationen sucht, oder die mir obliegenden Pflichten hinsichtlich der Prüfungsleistung für mich ganz oder teilweise erledigt. Ich habe die Dissertation in dieser oder -

In Vivo Real-Time Dynamics of ATP and ROS Production in Axonal
Hameren et al. Acta Neuropathologica Communications (2019) 7:86 https://doi.org/10.1186/s40478-019-0740-4 RESEARCH Open Access In vivo real-time dynamics of ATP and ROS production in axonal mitochondria show decoupling in mouse models of peripheral neuropathies Gerben van Hameren1* , Graham Campbell1, Marie Deck1, Jade Berthelot1, Benoit Gautier1, Patrice Quintana1, Roman Chrast2,3 and Nicolas Tricaud1* Abstract Mitochondria are critical for the function and maintenance of myelinated axons notably through Adenosine triphosphate (ATP) production. A direct by-product of this ATP production is reactive oxygen species (ROS), which are highly deleterious for neurons. While ATP shortage and ROS levels increase are involved in several neurodegenerative diseases, it is still unclear whether the real-time dynamics of both ATP and ROS production in axonal mitochondria are altered by axonal or demyelinating neuropathies. To answer this question, we imaged and quantified mitochondrial ATP and hydrogen peroxide (H2O2) in resting or stimulated peripheral nerve myelinated axons in vivo, using genetically-encoded fluorescent probes, two-photon time-lapse and CARS imaging. We found that ATP and H2O2 productions are intrinsically higher in nodes of Ranvier even in resting conditions. Axonal firing increased both ATP and H2O2 productions but with different dynamics: ROS production peaked shortly and transiently after the stimulation while ATP production increased gradually for a longer period of time. In neuropathic MFN2R94Q mice, mimicking Charcot-Marie-Tooth 2A disease, defective mitochondria failed to upregulate ATP production following axonal activity. However, elevated H2O2 production was largely sustained. Finally, inducing demyelination with lysophosphatidylcholine resulted in a reduced level of ATP while H2O2 level soared. -

Roles of Sigma-1 Receptors on Mitochondrial Functions Relevant to Neurodegenerative Diseases Tzu-Yu Weng1,2, Shang-Yi Anne Tsai1 and Tsung-Ping Su1*
Weng et al. Journal of Biomedical Science (2017) 24:74 DOI 10.1186/s12929-017-0380-6 REVIEW Open Access Roles of sigma-1 receptors on mitochondrial functions relevant to neurodegenerative diseases Tzu-Yu Weng1,2, Shang-Yi Anne Tsai1 and Tsung-Ping Su1* Abstract The sigma-1 receptor (Sig-1R) is a chaperone that resides mainly at the mitochondrion-associated endoplasmic reticulum (ER) membrane (called the MAMs) and acts as a dynamic pluripotent modulator in living systems. At the MAM, the Sig-1R is known to play a role in regulating the Ca2+ signaling between ER and mitochondria and in maintaining the structural integrity of the MAM. The MAM serves as bridges between ER and mitochondria regulating multiple functions such as Ca2+ transfer, energy exchange, lipid synthesis and transports, and protein folding that are pivotal to cell survival and defense. Recently, emerging evidences indicate that the MAM is critical in maintaining neuronal homeostasis. Thus, given the specific localization of the Sig-1R at the MAM, we highlight and propose that the direct or indirect regulations of the Sig-1R on mitochondrial functions may relate to neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD) and amyotrophic lateral sclerosis (ALS). In addition, the promising use of Sig-1R ligands to rescue mitochondrial dysfunction-induced neurodegeneration is addressed. Keywords: Sigma-1 receptor, Mitochondria, Mitochondrion-associated ER membrane (MAM), Neurodegenerative disorders Background also regulates Ca2+ influx by attenuating the coupling of The sigma-1 receptor (Sig-1R) is an endoplasmic the ER Ca2+ sensor STIM1 to Orai1 [3]. -

Pioneer Growth Cone Morphologies Reveal Proximal Increases in Substrate Affinity Within Leg Segments of Grasshopper Embryos
The Journal of Neuroscience February 1986, 6(2): 364-379 Pioneer Growth Cone Morphologies Reveal Proximal Increases in Substrate Affinity Within Leg Segments of Grasshopper Embryos Michael Gaudy* and David Bentley-f *Biophysics Group and tDepartment of Zoology, University of California, Berkeley, California 94720 We have compared the morphologies of approximately 5000 limb segment boundaries (Bentley and Caudy, 1983b) and antibody-labeled afferent pioneer growth cones fixed at various guidepost cells. The dominant mechanism appears to be the stages of growth along their characteristic path over the epithe- guidepost cells, a set of nonadjacent, axonless cell bodies of lium in the legs of grasshopper embryos, and have used growth immature neurons (Bentley and Caudy, 1983a, b; Bentley and cone morphology as an indicator of differences in the affinity of Keshishian, 1982a, b; Ho and Goodman, 1982; Keshishian and the epithelial substrate for pioneer growth cones in viva. Growth Bentley, 1983a-c; Taghert et al., 1982). Ablation of the most cone morphologies differ markedly between different locations proximal guidepost cell pair has been shown to result in lack of in limb buds, and also in the same location in limbs at different normal growth (Bentley and Caudy, 1983a, b). stages of differentiation. Growth cones characteristically extend Additional mechanisms apparently guide the Ti 1 growth cones branches and lamellae circumferentially along segment bound- proximally before they contact any of the above cues (Bentley aries, and filopodia and lamellae are retained (or extended) and Caudy, 1983b). One possible external cue is an adhesion longer there. Where they contact a relatively well-differentiated gradient on the epithelial substrate over which the pioneer growth segment boundary, the growth cones also abruptly reorient cir- cones navigate (Bentley and Caudy, 1983b; Berlot and Good- cumferentially. -

The Cell Biology of Neuronal Navigation
review The cell biology of neuronal navigation Hong-jun Song* and Mu-ming Poo† * Molecular Neurobiology Laboratory, Salk Institute for Biological Studies, La Jolla, California 92037, USA; e-mail: [email protected] † Department of Molecular and Cell Biology, University of California, Berkeley, California 94720, USA; e-mail: [email protected] Morphogenesis of the nervous system requires the directed migration of postmitotic neurons to designated locations in the nervous system and the guidance of axon growth cones to their synaptic targets. Evidence suggests that both forms of navigation depend on common guidance molecules, surface receptors and signal transduction pathways that link receptor activation to cytoskeletal reorganization. Future challenges remain not only in identifying all the components of the signalling pathways, but also in understanding how these pathways achieve signal amplification and adaptation—two essential cellular processes for neuronal navigation. euronal navigation during early development is essential for face adhesion molecules have been shown to be important in neu- establishing the highly ordered cellular organization and spe- ronal navigation14. For example, axonal growth along ‘pioneering’ Ncific nerve connections in the nervous system1,2. In the devel- axons, ‘guidepost’ cells, or ‘labelled’ pathways in the developing oping nervous system, newly generated cortical neurons in the ven- embryos15,16 can be attributed to selective adhesion owing to the tricular zone migrate along the surface of radial glia fibres and set- presence of specific cell-adhesion molecules (CAMs) on the neu- tle in the cortical plate to form orderly layers of the cortex3,4. ronal surface14. The migration of postmitotic cortical neurons Neurons generated in subcortical structures also undergo long- along radial glia depends on the presence of specific neuronal sur- range tangential migration to the cortex to form dispersed popula- face proteins, such as astrotactin17 and integrins18,19.