Digestion of CHO, Fats, and Proteins
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Avian Crop Function–A Review
Ann. Anim. Sci., Vol. 16, No. 3 (2016) 653–678 DOI: 10.1515/aoas-2016-0032 AVIAN CROP function – A REVIEW* * Bartosz Kierończyk1, Mateusz Rawski1, Jakub Długosz1, Sylwester Świątkiewicz2, Damian Józefiak1♦ 1Department of Animal Nutrition and Feed Management, Poznań University of Life Sciences, Wołyńska 33, 60-637 Poznań, Poland 2Department of Animal Nutrition and Feed Science, National Research Institute of Animal Production, 32-083 Balice n. Kraków, Poland ♦Corresponding author: [email protected] Abstract The aim of this review is to present and discuss the anatomy and physiology of crop in different avian species. The avian crop (ingluvies) present in most omnivorous and herbivorous bird spe- cies, plays a major role in feed storage and moistening, as well as functional barrier for pathogens through decreasing pH value by microbial fermentation. Moreover, recent data suggest that this gastrointestinal tract segment may play an important role in the regulation of the innate immune system of birds. In some avian species ingluvies secretes “crop milk” which provides high nutri- ents and energy content for nestlings growth. The crop has a crucial role in enhancing exogenous enzymes efficiency (for instance phytase and microbial amylase,β -glucanase), as well as the activ- ity of bacteriocins. Thus, ingluvies may have a significant impact on bird performance and health status during all stages of rearing. Efficient use of the crop in case of digesta retention time is es- sential for birds’ growth performance. Thus, a functionality of the crop is dependent on a number of factors, including age, dietary factors, infections as well as flock management. -

Raptor Digestion Facts
Raptor Digestion Facts For birds that have a crop, food passes to the crop to soften or to just be stored temporarily. From there food goes to the stomachs. Owls do not have crops, all other raptors do. Food is stored in the crop on the way down, but not on the way up. Birds have two stomachs (in this order): A glandular stomach (the proventriculus) which digests food chemically A muscular stomach or gizzard (the ventriculus) that grinds food with the aid or grit. In many carnivorous species – hawks, for example, their glandular stomach is so highly acidic, it dissolves bones. The bearded vulture of Europe and China is said to have a stomach so acidic it can dissolve the whole of a cow’s vertebra in one or two days. Pellets are formed in the gizzard. A given bird’s pellet will be the size and shape of their gizzard. The gizzard of birds serves the same function as the teeth and strong jaws of mammals. The gizzard is most developed in birds that eat plant parts. Birds intentionally ingest grit to be kept in their gizzard to help grind food. They can prevent this grit from passing through the digestive system with the food, and remain in the gizzard. Birds prefer brightly colored grit. Such examples of grit found in birds include: quartz, granite, ruby, gold, fruit pits, coal, ground oyster shells, black lava, and lead shot form shotguns (this of course causes lead poisoning, which we do see a lot of at Willowbrook). Many other birds cough-up pellets, especially those which feed much on insects. -

Crop Problems
Text and photos: Monique de Vrijer Translation: Diana Hedrick CROP PROBLEMS The crop serves as a temporary ‘storage bin’ for the feed the chicken eats. It is often full during and at the end of the day and should normally be empty when the bird starts its day in the morning. Unfortunately ‘crop prob- lems’ are not an uncommon occurrence and presents as symptomatic with many dif- ferent illnesses and condi- tions. The Crop The crop is part of the digestive system and is an enlarged part of the esophagus at the foot of the neck where feed is (temporarily) stored and "moistened" with mucous and saliva from the mouth until the contents are moved into the stomach. A chicken has two stomachs, the proventriculus (the glandular stomach) and the gizzard or ventriculus (also called the muscular stomach). The feed is transported from the crop into the the glandular stomach which, as the name implies, is where secretory enzymes are added to the feed mass to aid in digestion; one of which is pepsin which in turn activates the stomach to produce hydrochloric (stomach) acid lowering the PH to aid in digestion and absorption of nutrients and also helps to dissolve the calcium grit (usually oyster shell) releasing the calcium in it. These enzyme-filled gastric juices infuse the feed as it moves onwards towards the gizzard (ventriculus or muscular stomach). The gizzard is a small organ and not able to deal with large quantities of feed. It is composed of two oval shaped and thick walled powerful muscles with a tough horny lining. -

Evaluating and Treating the Gastrointestinal System
CHAPTER 14 Evaluating and Treating the Gastrointestinal System STACEY GELIS, BS c, BVS c (Hons), MACVS c ( Avian Health) The avian gastrointestinal tract (GIT) has undergone a multitude of changes during evolution to become a unique anatomical and physiological structure when compared to other animal orders. On the one hand it has evolved to take advantage of the physical and chemi- cal characteristics of a wide variety of food types.1 On the other hand, it has had to do so within the limitations of the requirements for flight.2 To this end, birds have evolved a lightweight beak and muscular ventriculus, which replaces the heavy bone, muscular and dental structure characteristic of reptiles and mammals. The ventriculus and small intestine are the heaviest struc- tures within the gastrointestinal tract and are located near the bird’s centre of gravity within the abdomen. Greg J. Harrison Greg J. The overall length of the GIT is also less than that of a comparable mammal, another weight-saving flight adap- tation. Interestingly, these characteristics are still shared with the flightless species such as ratites and penguins. In addition, the actual digestive process needs to be rapid to support the high metabolic rate typical of flighted birds.3 Gastrointestinal adaptations to the wide range of ecolog- ical niches that birds occupy mean that birds can take advantage of a huge variety of foodstuffs. The GIT hence shows the greatest degree of diversity of all the organ systems between different avian taxa. However, the pres- sures of convergent evolution have also meant that many distantly related species have developed a similar gastrointestinal anatomy to take advantage of particular food niches.3,4 Examples of these will be presented in the discussion of each section of the GIT. -

Gastrointestinal Tract Morphometrics and Content of Commercial and Indigenous Chicken Breeds with Differing Ranging Profiles
animals Article Gastrointestinal Tract Morphometrics and Content of Commercial and Indigenous Chicken Breeds with Differing Ranging Profiles Joanna Marchewka 1,*, Patryk Sztandarski 1 , Zaneta˙ Zdanowska-S ˛asiadek 1, Dobrochna Adamek-Urba ´nska 2 , Krzysztof Damaziak 3, Franciszek Wojciechowski 1, Anja B. Riber 4 and Stefan Gunnarsson 5 1 Institute of Genetics and Animal Biotechnology, Polish Academy of Sciences, Jastrz˛ebiec, 05-552 Magdalenka, Poland; [email protected] (P.S.); [email protected] (Z.Z.-S.);˙ [email protected] (F.W.) 2 Department of Ichthyology and Biotechnology in Aquaculture, Institute of Animal Science, Warsaw University of Life Sciences, 02-786 Warsaw, Poland; [email protected] 3 Department of Animal Breeding, Faculty of Animal Breeding, Bioengineering and Conservation, Institute of Animal Science, Warsaw University of Life Sciences, 02-786 Warsaw, Poland; [email protected] 4 Department of Animal Science, Aarhus University, DK-8830 Aarhus, Tjele, Denmark; [email protected] 5 Department of Animal Environment and Health, Swedish University of Agricultural Sciences (SLU), S-532 23 Skara, Sweden; [email protected] * Correspondence: [email protected] Simple Summary: Differences in the range use by poultry exist on the individual or breed level, even if equal opportunity of outdoor access is provided. Birds reared with access to the pasture consume some of Citation: Marchewka, J.; Sztandarski, the material found outdoors, such as plants, insects, and stones. The frequency of outdoor range use may P.; Zdanowska-S ˛asiadek, Z.;˙ Adamek-Urba´nska,D.; Damaziak, K.; be associated with the ingested material and the development of the bird gut. -

Digestive System
Digestive system Meghan best Organs and their Functions Oesophagus- a tube that food passes through to get to the stomach. Crop- the crop allows for temporary storage for food, which lets the sparrow take its time with digestion, and helps soften the food before entering the stomach. Liver- the birds liver produces bile, which helps with the breaking down of food. It also aids with the absorption of nutrients Proventriculus- the proventriculus is the first part of the stomach begins the process of breaking down food using pepsin enzymes and hydrochloric acid Gizzard- this is the second part of the stomach, it uses small stones and sand the the bird picks up while eating to grind the food down until it's very fine. Organs and their Functions Small intestine- this is where the food is digested and absorbed. In the small intestine proteins and fats get broken down, and the nutrients are then absorbed through the intestinal membranes and then carried into the blood. Pancreas- the pancreas produces enzymes used in the proventriculus to break down food. It also aids with the absorption of nutrients. Large intestine- the tube where all the leftover waste travels through to reach the cloaca. Cloaca- the cloaca holds waste until the sparrow is able to release it. both feces and urine are released through the cloaca. Dietary Habits of a Sparrow A sparrow's diet consists mostly of grains,seeds,insects and sometimes discarded food found on city streets. Sparrows find their prey by visiting bright lights at night and following lawnmowers, they catch their prey by pouncing on them and using their beaks to pick them up and eat them. -

The Internal Anatomy of the Silverfish Otenclepisma Campbelli Barnhart and Lepisma Saccharina Linnaeus (Thysanura: Lepismatidae)
THE INTERNAL ANATOMY OF THE SILVERFISH OTENCLEPISMA CAMPBELLI BARNHART AND LEPISMA SACCHARINA LINNAEUS (THYSANURA: LEPISMATIDAE) DISSERTATION Presented in Pertial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By CLYDE STERLING BARNHART, SR., B.Sc., M.Sc The Ohio State University 1958 Approved byj Department PREFACE In 19^7 the writer began a study of the trachea- tion of a silverfish collected from the Main Library on the Ohio State University campus. This began under the direction of the late Dr. C. H. Kennedy, professor of Entomology, the Ohio State University, in his course on Insect anatomy. Professor Kennedy was Impressed with the minute detail with which the tracheation could be traced since this insect was so small. It was his interest and encouragement which prompted the writer to continue this work beyond the course and later to expand it into the more complete study embodied in this dissertation. The writer is grateful to the late professor Kennedy for his part in providing the original encouragement for this study. The writer wishes also to express his sincere gratitude to Dr. Donald J. Borror, professor of Entomo logy* The Ohio State University, for his helpful guidance and suggestions in bringing the work of this dissertation to completion. li TABLE CP CONTENTS Pege INTRODUCTION................................... 1 MATERIALS AND METHODS........................... 2 TIES RESPIRATORY SYSTEM.......................... 4 THE ALIMENTARY CANAL............................ 14 THE CENTRAL NERVOUS SYSTEM...................... 24 THE DORSAL VESSEL............................... 28 THE REPRODUCTIVE ORGANS......................... 32 ABBREVIATIONS USED ON FIGURES.................... 40 FIGURES........................................ 43 SUMMARY......................................... 65 BIBLIOGRAPHY.................................... 68 ill LIST OF ILLUSTRATIONS Figure Page 1 Tracheation of the head, thorax, and first abdominal segment of C . -

By W. ERNEST MILES,F.R.C.S., Hon
August, 1936 ANO-RECTAL FISTUL,2E 319, Postgrad Med J: first published as 10.1136/pgmj.12.130.319 on 1 August 1936. Downloaded from ANO-RECTAL FISTULAE. By W. ERNEST MILES, F.R.C.S., Hon.F.R.C.S.I., Hon.F.A.C.S. (Foreign Associate of the French Academy of Surgery; Consulting Surgeon to the Cancer Hospital; Surgeon to the Gordon Hospital for Diseases of the Rectum.) Ano-rectal fistulae present both the simplest and the most intricate problems that may confront the surgeon when he undertakes their surgical treatment. Fortunately the great majority can readily be cured by a simple surgical procedure but from time to time instances crop up which, from their complicated nature, tax the ingenuity and skill of the operator to the utmost and even then his efforts may fail to effect a complete cure. It is in such circumstances that a patient may be well advised to continue to endure the discomfort of a discharging sinus rather than run the risk of the permanent loss of control over the contents of the rectum that may result from repeated and extensive operations. Etiology. With the exception of fistulae produced by penetrating wounds from without and of those resulting from carcinoma or fibrous stricture of the rectum, every ano-rectal fistula is preceded by an abscess which is the outcome of infection by Protected by copyright. micro-organisms conveyed to the tissues, in which the abscess forms, chiefly by means of the lymphatics. Cultures made from the pus in such abscesses reveal that the organisms concerned are, staphylococci, streptococci, the Bacillus coli communis, the Bacillus proteus and occasionally the tubercle bacillus. -

Drinking Mechanisms in the Zebra Finch and the Bengalese Finch’
THE CONDOR A JOURNAL OF AVIAN BIOLOGY I_\! Volume 92 Number 1 February 1990 MAROh WI0 The Condor 92: 1-28 Q The Cooper Ornithological Society 1990 WVERSITY OF ~[4br-fJ- DRINKING MECHANISMS IN THE ZEBRA FINCH AND THE BENGALESE FINCH’ J. HEIDWEILLER AND G. A. ZWEERS~ Department of NeurobehavioralMorphology, Zoological Laboratory, Universityof Leiden, The Netherlands Abstract. Two kinds of drinking behavior were studied by film and radiogram analysis of tip down drinking Zebra Finches (Poephilaguttata) and tip up drinking BengaleseFinches (Lonchurastriata) which use similar scoopingtongue motions to carry water into the mouth. Water transport through the pharynx differs: the Zebra Finch usesa scoopingmotion of the larynx that reoccurs in every motion cycle, while the beak is kept down. The Bengalese Finch elevates the head allowing water to flow downward due to gravity and pharyngeal properistalsis.Extensive analysesshow the anatomy of the speciesto be highly similar. The Zebra Finch is able to drink by a double scoop mechanism, because-unlike the Bengalese Finch-reflexes for glottis closure and esophagealperistalsis are used. Integration of these reflexesand a shift in timing of the larynx-scoophas modified tip up into tip down drinking. Thus, tip down is more complex than tip up drinking, since here actions from different cyclesand patterns are integrated in one motion cycle. Increased kinematic complexity is, apart from any historical scenario,an argumentthat tip down is derived from tip up drinking in Estrildidae. An evolutionary scenario is presented in which developments of scooping anatomical elements are seen as preadaptations.These developed by selectionon elements serving the highly specialized kind of feeding on seedsof Gramineae under high predator pressurein open fields, and allowed a wide secondaryextension of the feeding area. -
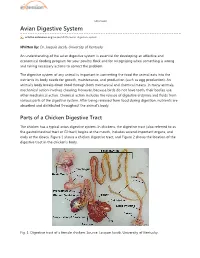
Avian Digestive System
eXtension Avian Digestive System articles.extension.org/pages/65376/avian-digestive-system Written by: Dr. Jacquie Jacob, University of Kentucky An understanding of the avian digestive system is essential for developing an effective and economical feeding program for your poultry flock and for recognizing when something is wrong and taking necessary actions to correct the problem. The digestive system of any animal is important in converting the food the animal eats into the nutrients its body needs for growth, maintenance, and production (such as egg production). An animal's body breaks down food through both mechanical and chemical means. In many animals, mechanical action involves chewing; however, because birds do not have teeth, their bodies use other mechanical action. Chemical action includes the release of digestive enzymes and fluids from various parts of the digestive system. After being released from food during digestion, nutrients are absorbed and distributed throughout the animal's body. Parts of a Chicken Digestive Tract The chicken has a typical avian digestive system. In chickens, the digestive tract (also referred to as the gastrointestinal tract or GI tract) begins at the mouth, includes several important organs, and ends at the cloaca. Figure 1 shows a chicken digestive tract, and Figure 2 shows the location of the digestive tract in the chicken's body. Fig. 1. Digestive tract of a female chicken. Source: Jacquie Jacob, University of Kentucky. Fig. 2. Location of the digestive tract in a female chicken. Source: Public domain. Beak/Mouth As with most birds, a chicken obtains feed by using its beak. Food picked up by the beak enters the mouth. -

The Digestive System of the Carolina Locust (Dissosteira Carolina Linn.) Harrison Morton Tietz University of Massachusetts Amherst
University of Massachusetts Amherst ScholarWorks@UMass Amherst Masters Theses 1911 - February 2014 1922 The digestive system of the Carolina locust (Dissosteira carolina Linn.) Harrison Morton Tietz University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/theses Tietz, Harrison Morton, "The digestive system of the Carolina locust (Dissosteira carolina Linn.)" (1922). Masters Theses 1911 - February 2014. 2033. Retrieved from https://scholarworks.umass.edu/theses/2033 This thesis is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Masters Theses 1911 - February 2014 by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. •1 s I DATE DUE UNIVERSITY OF MASSACHUSETTS LIBRARY MORE ID -3234 HZ68 1923' T564 . TEE DIGESTIVE SYSTEM OF THE C^ROLUJA LOCUST (Diesosteira Carolina Linn.) HARRISOH M. TIETZ B. Sc. Submitted -^a a Thesis for the Degree of Master of Science MASSACHUSETTS AGRICULTURAL COLLIDE AMHERST , MASS 1922 ) THB AH TOUT OP MS DIGESTIVE SYSTEM 0? THE CAROLINA LOCUST (Dissosteira oarolina, Linn. By Harrison M.TIetz. Introduction. The typioal inaoot dissected by most students in biolopy Is the Carolina looust or some other common grasshopper. Many laboratory manuals describe the anatomy of these orthop- terons in more or leas detail but with tto few illustrations. This laok of illustrntions makes it diffioult for the instructor to present the subject and leads to confusion in the minds of the tudents. It is the aim of this paper to meet this need in part by presenting in its text and figures the results of a detailed study of the alimentary tract of the Carolina looust. -

Breed and Sex Differences in the Gross Anatomy, Digesta Ph And
Brazilian Journal of Poultry Science Revista Brasileira de Ciência Avícola Breed and Sex Differences in the Gross Anatomy, ISSN 1516-635X Apr - Jun 2017 / v.19 / n.2 / 339-346 Digesta pH and Histomorphology of the Gastrointestinal Tract of Gallus Gallus Domesticus http://dx.doi.org/10.1590/1806-9061-2016-0275 Author(s) ABSTRACT Mabelebele MI A study was conducted to investigate the influence of breed and Norris DII sex in the gross anatomy, digesta and histology of Ross 308 broiler and Brown DII Ginindza MMII Venda chickens. Chickens were slaughtered at 90 days of age and the Ngambi JWII pH of the digestive organs was measured immediately after slaughter. The digestive organ weights and lengths of Ross 308 broiler and Venda I University of South Africa, College of Agri- chickens were measured. Tissue samples of the duodenum, ileum and culture and Environmental Science, Florida jejunal from each treatment group were collected and histologically Campus, Johannesburg, South Africa II University of Limpopo, School of Agricul- examined. Higher (p<0.05) gizzard pH values were observed in male tural and Environmental Sciences, Depart- and female of Ross 308 broiler and Venda chickens. The jejunal and ment of Animal Science, Polokwane, South Africa ileal pH values were lower (p<0.05) for Venda chickens than in Ross 308 broiler chickens. The absolute weights of the gastrointestinal tract, crop, proventriculus and gizzard were lighter (p<0.05) in Venda chickens than in Ross 308 broiler chickens. The relative organ weights of the GIT, proventriculus, gizzard and caeca were higher (p<0.05) in Venda chickens than in Ross 308 broiler chickens aged 90 days.