Study on Aspergillus Species and Aflatoxin Levels in Sorghum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
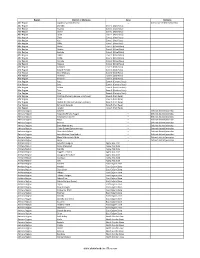
Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

ETHIOPIA: MENINGITIS EPIDEMIC 2001 This Final Report Is Intended for Reporting on Emergency Appeals Appeal No
3 December, ETHIOPIA: MENINGITIS EPIDEMIC 2001 This Final Report is intended for reporting on emergency appeals Appeal No. 12/01 Launched on: March 2001 for 3 months for CHF 1,006,787 DREF Allocated: CHF 200,000 (entirely reimbursed) Beneficiaries: 1.5 million Period covered: March - October 2001; last Operations Update (no. 2) issued on 11 June 2001) “At a glance” Appeal coverage: 91% Related Appeals: 01.13/2001 - 2001 Ethiopia annual Summary/Update: This programme, launched to assist the Ethiopian Red Cross Society (ERCS) to respond to a meningitis outbreak, has achieved the intended objectives. A final payment from ECHO remains pending but is expected shortly, and this will cover the small deficit currently reflected in the attached final financial report. Operational Developments: Ethiopia lies in the African Meningitis Belt which extends from the Red Sea in the east to the Atlantic ocean in the west. It has suffered major epidemics of meningococcal meningitis, a bacteria disease of the central nervous system, in 1981 when there were 50,000 cases and nearly a thousand deaths and in 1989 when there 46,000 cases and 1,700 deaths. Between June and August in 2000, there was an outbreak in Addis Ababa with over 850 cases and 33 deaths. Major epidemics usually occur every 8 to 12 years. This epidemic started in October 2000 in Quarit Woreda in West Gojam in Amhara Region and over a period of several months spread throughout the country. It reached its peak in week 22 of the epidemic (5-11 March 2001) when 724 cases were reported although a decrease in the number of cases week on week was reversed in week 29 (23-29 April 2001) when a lower peak was reached. -

Protecting Land Tenure Security of Women in Ethiopia: Evidence from the Land Investment for Transformation Program
PROTECTING LAND TENURE SECURITY OF WOMEN IN ETHIOPIA: EVIDENCE FROM THE LAND INVESTMENT FOR TRANSFORMATION PROGRAM Workwoha Mekonen, Ziade Hailu, John Leckie, and Gladys Savolainen Land Investment for Transformation Programme (LIFT) (DAI Global) This research paper was created with funding and technical support of the Research Consortium on Women’s Land Rights, an initiative of Resource Equity. The Research Consortium on Women’s Land Rights is a community of learning and practice that works to increase the quantity and strengthen the quality of research on interventions to advance women’s land and resource rights. Among other things, the Consortium commissions new research that promotes innovations in practice and addresses gaps in evidence on what works to improve women’s land rights. Learn more about the Research Consortium on Women’s Land Rights by visiting https://consortium.resourceequity.org/ This paper assesses the effectiveness of a specific land tenure intervention to improve the lives of women, by asking new questions of available project data sets. ABSTRACT The purpose of this research is to investigate threats to women’s land rights and explore the effectiveness of land certification interventions using evidence from the Land Investment for Transformation (LIFT) program in Ethiopia. More specifically, the study aims to provide evidence on the extent that LIFT contributed to women’s tenure security. The research used a mixed method approach that integrated quantitative and qualitative data. Quantitative information was analyzed from the profiles of more than seven million parcels to understand how the program had incorporated gender interests into the Second Level Land Certification (SLLC) process. -

Local History of Ethiopia Ma - Mezzo © Bernhard Lindahl (2008)
Local History of Ethiopia Ma - Mezzo © Bernhard Lindahl (2008) ma, maa (O) why? HES37 Ma 1258'/3813' 2093 m, near Deresge 12/38 [Gz] HES37 Ma Abo (church) 1259'/3812' 2549 m 12/38 [Gz] JEH61 Maabai (plain) 12/40 [WO] HEM61 Maaga (Maago), see Mahago HEU35 Maago 2354 m 12/39 [LM WO] HEU71 Maajeraro (Ma'ajeraro) 1320'/3931' 2345 m, 13/39 [Gz] south of Mekele -- Maale language, an Omotic language spoken in the Bako-Gazer district -- Maale people, living at some distance to the north-west of the Konso HCC.. Maale (area), east of Jinka 05/36 [x] ?? Maana, east of Ankar in the north-west 12/37? [n] JEJ40 Maandita (area) 12/41 [WO] HFF31 Maaquddi, see Meakudi maar (T) honey HFC45 Maar (Amba Maar) 1401'/3706' 1151 m 14/37 [Gz] HEU62 Maara 1314'/3935' 1940 m 13/39 [Gu Gz] JEJ42 Maaru (area) 12/41 [WO] maass..: masara (O) castle, temple JEJ52 Maassarra (area) 12/41 [WO] Ma.., see also Me.. -- Mabaan (Burun), name of a small ethnic group, numbering 3,026 at one census, but about 23 only according to the 1994 census maber (Gurage) monthly Christian gathering where there is an orthodox church HET52 Maber 1312'/3838' 1996 m 13/38 [WO Gz] mabera: mabara (O) religious organization of a group of men or women JEC50 Mabera (area), cf Mebera 11/41 [WO] mabil: mebil (mäbil) (A) food, eatables -- Mabil, Mavil, name of a Mecha Oromo tribe HDR42 Mabil, see Koli, cf Mebel JEP96 Mabra 1330'/4116' 126 m, 13/41 [WO Gz] near the border of Eritrea, cf Mebera HEU91 Macalle, see Mekele JDK54 Macanis, see Makanissa HDM12 Macaniso, see Makaniso HES69 Macanna, see Makanna, and also Mekane Birhan HFF64 Macargot, see Makargot JER02 Macarra, see Makarra HES50 Macatat, see Makatat HDH78 Maccanissa, see Makanisa HDE04 Macchi, se Meki HFF02 Macden, see May Mekden (with sub-post office) macha (O) 1. -

Next Stage for Dairy Development in Ethiopia
The NEXT STAGE IN DAIRY DEVELOPMENT FOR ETHIOPIA Dairy Value Chains, End Markets and Food Security Cooperative Agreement 663-A-00-05-00431-00 Submitted by Land O'Lakes, Inc. P.O. Box 3099 code 1250, Addis Ababa, Ethiopia November 2010 2 TABLE OF CONTENT Pages ACRONYMNS…………………………………………………………………………………. 5 EXECUTIVE SUMMARY …………………………………………………………………... 6 1. OVERVIEW OF THE DAIRY SUB-SECTOR STUDY………………………………….10 1.1. The Role of the Dairy Sub-Sector in the Economy of Ethiopia 1.1.1. Milk Production and its Allocation 1.1.2 Livestock and Milk in the household economy 1.2. The Challenges 1.3. A Value Chain Approach 1.4. The Tasks and the Study Team 2. DEMAND FOR MILK AND MILK PRODUCTS…………………………………….…. 15 2.1. Milk Consumption 2.1.1. Milk and Milk Product Consumption in Urban Areas 2.1.2. Milk and Milk Product Consumption in Rural Areas 2.1.3. Milk and Milk Product Consumption in Pastoral Areas 2.2. Milk Consumption Compared to Other Countries 2.3. Milk’s Role for Food Security and Household Nutrition 2.4. Consumption of Imported Milk Products by Areas and Product Categories – domestic and imported 2.5. Milk Consumption in 2020 2.5.1.. High Estimate 2.5.2. Middle of the Range Estimate 2.5.3. Low Estimate 2.6. Assessment 3. DAIRY PRODUCTION……………………………………………………………..…… 30 3.1. Current Situation 3.2. Milk Production Areas (waiting on the maps) 3.3. Production systems and Milk Sheds (see zonal data in annex 3.3.1. Commercial Production 3.3.2. Peri-Urban and Urban Production 3.3.3. -
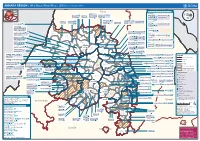
AMHARA REGION : Who Does What Where (3W) (As of 13 February 2013)
AMHARA REGION : Who Does What Where (3W) (as of 13 February 2013) Tigray Tigray Interventions/Projects at Woreda Level Afar Amhara ERCS: Lay Gayint: Beneshangul Gumu / Dire Dawa Plan Int.: Addis Ababa Hareri Save the fk Save the Save the df d/k/ CARE:f k Save the Children:f Gambela Save the Oromia Children: Children:f Children: Somali FHI: Welthungerhilfe: SNNPR j j Children:l lf/k / Oxfam GB:af ACF: ACF: Save the Save the af/k af/k Save the df Save the Save the Tach Gayint: Children:f Children: Children:fj Children:l Children: l FHI:l/k MSF Holand:f/ ! kj CARE: k Save the Children:f ! FHI:lf/k Oxfam GB: a Tselemt Save the Childrenf: j Addi Dessie Zuria: WVE: Arekay dlfk Tsegede ! Beyeda Concern:î l/ Mirab ! Concern:/ Welthungerhilfe:k Save the Children: Armacho f/k Debark Save the Children:fj Kelela: Welthungerhilfe: ! / Tach Abergele CRS: ak Save the Children:fj ! Armacho ! FHI: Save the l/k Save thef Dabat Janamora Legambo: Children:dfkj Children: ! Plan Int.:d/ j WVE: Concern: GOAL: Save the Children: dlfk Sahla k/ a / f ! ! Save the ! Lay Metema North Ziquala Children:fkj Armacho Wegera ACF: Save the Children: Tenta: ! k f Gonder ! Wag WVE: Plan Int.: / Concern: Save the dlfk Himra d k/ a WVE: ! Children: f Sekota GOAL: dlf Save the Children: Concern: Save the / ! Save: f/k Chilga ! a/ j East Children:f West ! Belesa FHI:l Save the Children:/ /k ! Gonder Belesa Dehana ! CRS: Welthungerhilfe:/ Dembia Zuria ! î Save thedf Gaz GOAL: Children: Quara ! / j CARE: WVE: Gibla ! l ! Save the Children: Welthungerhilfe: k d k/ Takusa dlfj k -

Debre Berhan University College of Social Sciences & Humanities Department of Geography and Environmental Studies
DEBRE BERHAN UNIVERSITY COLLEGE OF SOCIAL SCIENCES & HUMANITIES DEPARTMENT OF GEOGRAPHY AND ENVIRONMENTAL STUDIES IMPACTS OF CLIMATE CHANGE AND VARIABILITY ON RURAL LIVELIHOODS AND COMMUNITY RESPONSES: THE CASE OF MERHABETE WOREDA, NORTH SHEWA ZONE, AMHARA NATIONAL REGIONAL STATE, ETHIOPIA. By: Kefelegn Chernet July 2020 Debre Berhan, Ethiopia DEBRE BERHAN UNIVERSITY COLLEGE OF SOCIAL SCIENCES & HUMANITIES DEPARTMENT OF GEOGRAPHY AND ENVIRONMENTAL STUDIES IMPACTS OF CLIMATE CHANGE AND VARIABILITY ON RURAL LIVELIHOODS AND COMMUNITY RESPONSES: THE CASE OF MERHABETE WOREDA, NORTH SHEWA ZONE, AMHARA NATIONAL REGIONAL STATE, ETHIOPIA. By Kefelegn Chernet A Thesis Submitted to the Department of Geography and Environmental Studies to Presented in Partial fulfillment of the requirement for the Degree of Master of science in Environment and Sustainable Development. Advisor Dr. Arragaw Alemayehu Debre Berhan University Debre Berhan, Ethiopia July, 2020 DEBRE BERHAN UNIVERSITY COLLEGE OF SOCIAL SCIENCES & HUMANITIES DEPARTMENT OF GEOGRAPHY AND ENVIRONMENTAL STUDIES THESIS SUBMISSION FOR DEFENSE APPROVAL SHEET – I This is to certify that the thesis entitled: Impacts of climate change and variability on rural livelihoods and community responses: the case of Merhabete woreda, North shewa zone, Amhara national regional state, Ethiopia. submitted in partial fulfillment of the requirements for the degree of Masters of Science with specialization in Environment and sustainable development of the Graduate Program of the Geography and Environmental studies, College of Social Science and Humanities, Debre Berhan University and is a record of original research carried out by Kefelegn Chernet Id. No PGR 028/11, under my supervision, and no part of the thesis has been submitted for any other degree or diploma. -

English-Full (0.5
Enhancing the Role of Forestry in Building Climate Resilient Green Economy in Ethiopia Strategy for scaling up effective forest management practices in Amhara National Regional State with particular emphasis on smallholder plantations Wubalem Tadesse Alemu Gezahegne Teshome Tesema Bitew Shibabaw Berihun Tefera Habtemariam Kassa Center for International Forestry Research Ethiopia Office Addis Ababa October 2015 Copyright © Center for International Forestry Research, 2015 Cover photo by authors FOREWORD This regional strategy document for scaling up effective forest management practices in Amhara National Regional State, with particular emphasis on smallholder plantations, was produced as one of the outputs of a project entitled “Enhancing the Role of Forestry in Ethiopia’s Climate Resilient Green Economy”, and implemented between September 2013 and August 2015. CIFOR and our ministry actively collaborated in the planning and implementation of the project, which involved over 25 senior experts drawn from Federal ministries, regional bureaus, Federal and regional research institutes, and from Wondo Genet College of Forestry and Natural Resources and other universities. The senior experts were organised into five teams, which set out to identify effective forest management practices, and enabling conditions for scaling them up, with the aim of significantly enhancing the role of forests in building a climate resilient green economy in Ethiopia. The five forest management practices studied were: the establishment and management of area exclosures; the management of plantation forests; Participatory Forest Management (PFM); agroforestry (AF); and the management of dry forests and woodlands. Each team focused on only one of the five forest management practices, and concentrated its study in one regional state. -

Ethiopia: Amhara Region Administrative Map (As of 05 Jan 2015)
Ethiopia: Amhara region administrative map (as of 05 Jan 2015) ! ! ! ! ! ! ! ! ! ! Abrha jara ! Tselemt !Adi Arikay Town ! Addi Arekay ! Zarima Town !Kerakr ! ! T!IGRAY Tsegede ! ! Mirab Armacho Beyeda ! Debark ! Debarq Town ! Dil Yibza Town ! ! Weken Town Abergele Tach Armacho ! Sanja Town Mekane Berhan Town ! Dabat DabatTown ! Metema Town ! Janamora ! Masero Denb Town ! Sahla ! Kokit Town Gedebge Town SUDAN ! ! Wegera ! Genda Wuha Town Ziquala ! Amba Giorges Town Tsitsika Town ! ! ! ! Metema Lay ArmachoTikil Dingay Town ! Wag Himra North Gonder ! Sekota Sekota ! Shinfa Tomn Negade Bahr ! ! Gondar Chilga Aukel Ketema ! ! Ayimba Town East Belesa Seraba ! Hamusit ! ! West Belesa ! ! ARIBAYA TOWN Gonder Zuria ! Koladiba Town AMED WERK TOWN ! Dehana ! Dagoma ! Dembia Maksegnit ! Gwehala ! ! Chuahit Town ! ! ! Salya Town Gaz Gibla ! Infranz Gorgora Town ! ! Quara Gelegu Town Takusa Dalga Town ! ! Ebenat Kobo Town Adis Zemen Town Bugna ! ! ! Ambo Meda TownEbinat ! ! Yafiga Town Kobo ! Gidan Libo Kemkem ! Esey Debr Lake Tana Lalibela Town Gomenge ! Lasta ! Muja Town Robit ! ! ! Dengel Ber Gobye Town Shahura ! ! ! Wereta Town Kulmesk Town Alfa ! Amedber Town ! ! KUNIZILA TOWN ! Debre Tabor North Wollo ! Hara Town Fogera Lay Gayint Weldiya ! Farta ! Gasay! Town Meket ! Hamusit Ketrma ! ! Filahit Town Guba Lafto ! AFAR South Gonder Sal!i Town Nefas mewicha Town ! ! Fendiqa Town Zege Town Anibesema Jawi ! ! ! MersaTown Semen Achefer ! Arib Gebeya YISMALA TOWN ! Este Town Arb Gegeya Town Kon Town ! ! ! ! Wegel tena Town Habru ! Fendka Town Dera -

AMHARA Demography and Health
1 AMHARA Demography and Health Aynalem Adugna January 1, 2021 www.EthioDemographyAndHealth.Org 2 Amhara Suggested citation: Amhara: Demography and Health Aynalem Adugna January 1, 20201 www.EthioDemographyAndHealth.Org Landforms, Climate and Economy Located in northwestern Ethiopia the Amhara Region between 9°20' and 14°20' North latitude and 36° 20' and 40° 20' East longitude the Amhara Region has an estimated land area of about 170000 square kilometers . The region borders Tigray in the North, Afar in the East, Oromiya in the South, Benishangul-Gumiz in the Southwest and the country of Sudan to the west [1]. Amhara is divided into 11 zones, and 140 Weredas (see map at the bottom of this page). There are about 3429 kebeles (the smallest administrative units) [1]. "Decision-making power has recently been decentralized to Weredas and thus the Weredas are responsible for all development activities in their areas." The 11 administrative zones are: North Gonder, South Gonder, West Gojjam, East Gojjam, Awie, Wag Hemra, North Wollo, South Wollo, Oromia, North Shewa and Bahir Dar City special zone. [1] The historic Amhara Region contains much of the highland plateaus above 1500 meters with rugged formations, gorges and valleys, and millions of settlements for Amhara villages surrounded by subsistence farms and grazing fields. In this Region are located, the world- renowned Nile River and its source, Lake Tana, as well as historic sites including Gonder, and Lalibela. "Interspersed on the landscape are higher mountain ranges and cratered cones, the highest of which, at 4,620 meters, is Ras Dashen Terara northeast of Gonder. -

Periodic Monitoring Report Working 2016 Humanitarian Requirements Document – Ethiopia Group
DRMTechnical Periodic Monitoring Report Working 2016 Humanitarian Requirements Document – Ethiopia Group Covering 1 Jan to 31 Dec 2016 Prepared by Clusters and NDRMC Introduction The El Niño global climactic event significantly affected the 2015 meher/summer rains on the heels of failed belg/ spring rains in 2015, driving food insecurity, malnutrition and serious water shortages in many parts of the country. The Government and humanitarian partners issued a joint 2016 Humanitarian Requirements Document (HRD) in December 2015 requesting US$1.4 billion to assist 10.2 million people with food, health and nutrition, water, agriculture, shelter and non-food items, protection and emergency education responses. Following the delay and erratic performance of the belg/spring rains in 2016, a Prioritization Statement was issued in May 2016 with updated humanitarian requirements in nutrition (MAM), agriculture, shelter and non-food items and education.The Mid-Year Review of the HRD identified 9.7 million beneficiaries and updated the funding requirements to $1.2 billion. The 2016 HRD is 69 per cent funded, with contributions of $1.08 billion from international donors and the Government of Ethiopia (including carry-over resources from 2015). Under the leadership of the Government of Ethiopia delivery of life-saving and life- sustaining humanitarian assistance continues across the sectors. However, effective humanitarian response was challenged by shortage of resources, limited logistical capacities and associated delays, and weak real-time information management. This Periodic Monitoring Report (PMR) provides a summary of the cluster financial inputs against outputs and achievements against cluster objectives using secured funding since the launch of the 2016 HRD. -

GAFSP Proposal Is Intended to Fill the Financing Gap Required for the Implementation of AGP-II and Thereby Help Attain the PDO
THE FEDERAL DEMOCRACTIC REPUBLIC OF ETHIOPIA MINISTRY OF FINANCE AND ECONOMIC COOPERATION IN COLLABORATION WITH MINISTRY OF AGRICULTURE AND NATURAL RESOURCES GLOBAL AGRICULTURE AND FOOD SECURITY PROGRAMME REQUEST FOR FUNDING PUBLIC SECTOR WINDOW SECOND AGRICULTURAL GROWTH PROGRAM (AGP-II GAP FINANCING) IN THE AMOUNT OF US$ 55.3 MILLION PART I: SUMMARY OF AGRICULTURE AND FOOD SECURITY STRATEGY AND ASSOCIATED INVESTMENT PLAN PART II: ETHIOPIA PROPOSAL FOR GAFSP CO-FINANCING 30 December 2016 TABLE OF CONTENTS CONTENTS ..................................................................................................................................................................... I ABBREVIATIONS AND ACRONYMS ............................................................................................................................ II PART I: SUMMARY OF OVERALL AGRICULTURE AND FOOD SECURITY STRATEGY AND ASSOCIATED INVESTMENT PLAN ....................................................................................................................................... 1 1.1 OVERALL SECTOR STRATEGY AND INVESTMENT PLAN AND PAST PERFORMANCE ............................. 1 1.2 KEY ELEMENTS OF THE POLICY ENVIRONMENT.......................................................................................... 3 1.3 GOVERNMENT COMMITMENT TO AGRICULTURE, FOOD AND NUTRITION SECURITY ............................ 6 14 STRATEGY AND INVESTMENT PLAN DEVELOPMENT PROCESS AND UPDATE ........................................ 7 1.5 IMPLEMENTATION ARRANGEMENTS AND CAPACITY