Hellenic Plant Protection Journal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Observational Evidence on the Effects of Mega-Fires on the Frequency Of
Science of the Total Environment 592 (2017) 262–276 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Observational evidence on the effects of mega-fires on the frequency of hydrogeomorphic hazards. The case of the Peloponnese fires of 2007 in Greece Diakakis M. a,⁎, Nikolopoulos E.I. b,MavroulisS.a,VassilakisE.a,KorakakiE.c a Faculty of Geology and Geoenvironment, National & Kapodistrian University of Athens, Panepistimioupoli, Zografou GR15784, Greece b Department of Civil and Environmental Engineering, University of Connecticut, Storrs, CT, USA c WWF Greece, 21 Lembessi St., 117 43 Athens, Greece HIGHLIGHTS GRAPHICAL ABSTRACT • The mega fire of 2007 in Greece and its effects of hydrogeomorphic events are studied. • The frequency of such events over the period 1989–2016 is examined. • Results show an increase in floods by 3.3 times and mass movement events by 5.6. • Increase in frequency of such events is steeper in affected areas than unaf- fected. • Increases are found even in months that record a decrease in extreme rainfall. article info abstract Article history: Even though rare, mega-fires raging during very dry and windy conditions, record catastrophic impacts on infra- Received 6 January 2017 structure, the environment and human life, as well as extremely high suppression and rehabilitation costs. Apart Received in revised form 7 March 2017 from the direct consequences, mega-fires induce long-term effects in the geomorphological and hydrological Accepted 8 March 2017 processes, influencing environmental factors that in turn can affect the occurrence of other natural hazards, Available online xxxx such as floods and mass movement phenomena. -

Ganas, A., Serpelloni, E., Drakatos, G., Kolligri, M., Adamis, I., Tsimi, Ch
This article was downloaded by: [HEAL-Link Consortium] On: 20 October 2009 Access details: Access Details: [subscription number 772810551] Publisher Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Journal of Earthquake Engineering Publication details, including instructions for authors and subscription information: http://www.informaworld.com/smpp/title~content=t741771161 The Mw 6.4 SW-Achaia (Western Greece) Earthquake of 8 June 2008: Seismological, Field, GPS Observations, and Stress Modeling A. Ganas a; E. Serpelloni b; G. Drakatos a; M. Kolligri a; I. Adamis a; Ch. Tsimi a; E. Batsi a a Institute of Geodynamics, National Observatory of Athens, Athens, Greece b Istituto Nazionale di Geofisica e Vulcanologia, Centro Nazionale Terremoti, Bologna, Italy Online Publication Date: 01 December 2009 To cite this Article Ganas, A., Serpelloni, E., Drakatos, G., Kolligri, M., Adamis, I., Tsimi, Ch. and Batsi, E.(2009)'The Mw 6.4 SW- Achaia (Western Greece) Earthquake of 8 June 2008: Seismological, Field, GPS Observations, and Stress Modeling',Journal of Earthquake Engineering,13:8,1101 — 1124 To link to this Article: DOI: 10.1080/13632460902933899 URL: http://dx.doi.org/10.1080/13632460902933899 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. -
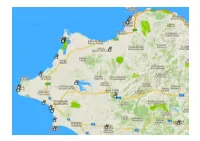
22-Nord-West.Pdf
Ort Wo Koordinaten Beschrieb Patras-1 (Valtos) (Richt. Athen). 38.28111, 21.75111 Beachside parking to the north of Patras. Midway between Patras and the Rio Bridge. Approach from SW (Patras) only, as there are "No Entry" signs from East. Beach bars with water taps. Alte Küststrasse fahren Acropolis-Travel Patras. 38.25918, 21.73874 Acropolis Travel Patras – Reisebüro ist neu hier 38.259186 21.738749 Iroon Polytechneioy & 1 Thessalonilkis Srt Patras 264 41 Griechenland Tel. +30 697 329 1605 Mail: Acropolis Travel <[email protected]> Patras-2 (Marina). 38.26308, 21.7389 Overnight parking on marina road which runs parallel to main road on sea side. Parking in various spots mostly at N end. Water taps all along face of quay wall towards the boats. Noisy. Lit. Bars nearby and excellent restaurant called Navpigeio (?????????) on opposite side of main road. To the left of the Hilti showroom. Patras-3 Gas füllen Nähe Patras. 38.10416, 21.63555 Ob das heute noch möglich ist ?? Kalogria-1 (Camper-Stop) (10€) 38.15968, 21.37158 V+E, Strom, WiFi usw. 10€. Nur 1 Std. nach Patras Kalogria-2 ( Beach Mid). 38.15223, 21.36885 Just to the south of the main wildcamping spot. Quieter and closer to the beach. Kalogria-3 (Beach North) 38.15659, 21.36793 Huge Parking area next to lovely sandy beach. Good taverna just along the road. WoMo-Kollege "Toni" warnt: Ja - die beiden Plätze Kalogria Nord und Mid sind aus meiner Sicht nicht mehr zu gebrauchen. Leider. Beide waren sehr beliebt. Es gab mehrere Vorkommnisse mit Belästigung und Diebstahl durch Zigeuner. -

UN/LOCODE) for Greece
United Nations Code for Trade and Transport Locations (UN/LOCODE) for Greece N.B. To check the official, current database of UN/LOCODEs see: https://www.unece.org/cefact/locode/service/location.html UN/LOCODE Location Name State Functionality Status Coordinatesi GR 2NR Neon Rysion 54 Road terminal; Recognised location 4029N 02259E GR 5ZE Zervochórion 32 Road terminal; Recognised location 3924N 02033E GR 6TL Lití 54 Road terminal; Recognised location 4044N 02258E GR 9OP Dhílesi 03 Road terminal; Recognised location 3821N 02340E GR A8A Anixi A1 Road terminal; Recognised location 3808N 02352E GR AAI Ágioi Anárgyroi 31 Multimodal function, ICD etc.; Recognised location 3908N 02101E GR AAR Acharnes A1 Multimodal function, ICD etc.; Recognised location 3805N 02344E GR AAS Ágios Athanásios 54 Road terminal; Recognised location 4043N 02243E GR ABD Abdera 72 Road terminal; Recognised location 4056N 02458E GR ABO Ambelókipoi Road terminal; Recognised location 4028N 02118E GR ACH Akharnaí A1 Rail terminal; Road terminal; Approved by national 3805N 02344E government agency GR ACL Achladi Port; Code adopted by IATA or ECLAC 3853N 02249E GR ADA Amaliada 14 Road terminal; Recognised location 3748N 02121E GR ADI Livádia 31 Road terminal; Recognised location 3925N 02106E GR ADK Ano Diakopto 13 Road terminal; Recognised location 3808N 02214E GR ADL Adamas Milos 82 Port; Request under consideration 3643N 02426E GR ADO Áhdendron 54 Rail terminal; Road terminal; Recognised location 4040N 02236E GR AEF Agia Efimia Port; Code adopted by IATA or ECLAC 3818N -

Irrigation Practice in the Region of Western Greece Καταγραφή
Efficient Irrigation Management Tools for Agricultural Cultivations and Urban Landscapes IRMA Irriga tion practice Καταγραφή in the Region of αρδευτικής πρακτικής Western Greece στην WP4, Action 4.2. Del. 4.2.1 Περιφέρεια Interviews and report of the survey outcomes on Δυτικής irrigation practices Ελλάδας www.irrigation-management.eu Front page back [intentionally left blank] IRMA info European Territorial Cooperation Programmes (ETCP) GREECE-ITALY 2007-2013 www.greece-italy.eu Efficient Irrigation Management Tools for Agricultural Cultivations and Urban Landscapes (IRMA) www.irrigation-management.eu 3 IRMA partners LP, Lead Partner, TEIEP Technological Educational Institution of Epirus http://www.teiep.gr, http://research.teiep.gr P2, AEPDE Olympiaki S.A., Development Enterprise of the Region of Western Greece http://www.aepde.gr P3, INEA / P7, CRA Ιnstituto Nazionale di Economia Agraria http://www.inea.it P4, ISPA-CNR Consiglio Nazionale delle Ricerche - Istituto di Scienze delle Produzioni Alimentari http://www.ispa.cnr.it/ P5, ROP Regione di Puglia http://www.regione.puglia.it P6, ROEDM Decentralised Administration of Epirus– Western Macedonia http://www.apdhp-dm.gov.gr 4 www.irrigation-management.eu WP4 Deliverable 4.2.1. Interviews and report of the survey outcomes on irrigation practices Involved partners: Headquarters: 23 Aegeou St. & Amerikis, 26441 Patras, GREECE T: 0030 2610 318224, 0030 2610 311872 Fax: 0030 2610 317877 Branch: 31 Manolopoulou St, 27100 Pyrgos, GREECE Τ: 0030 26210 37146, 0030 37194, 37223, Fax: 0030 26210 37169 e-mail: [email protected], website: www.aepde.gr Subcontractor Procurement 16 Team Patras 926 - 04/06/2015 ΑΔΑ: Ψ4Φ9465ΦΟΤ-ΔΗΡ Dr. Myriounis Christos Michalopoulos K. -

Selido3 Part 1
Δελτίο της Ελληνικής Γεωλογικής Εταιρίας, 2010 Bulletin of the Geological Society of Greece, 2010 Πρακτικά 12ου Διεθνούς Συνεδρίου Proceedings of the 12th International Congress Πάτρα, Μάιος 2010 Patras, May, 2010 ENGINEERING GEOLOGICAL AND GEOTECHNICAL INVESTIGATION OF LANDSLIDE EVENTS IN WILDFIRE AFFECTED AREAS OF ILIA PREFECTURE, WESTERN GREECE Depountis N.1, Lainas S.2, Pyrgakis D.2, Sabatakakis N.2, and Koukis G.2 1 Region of Western Greece, Directorate of Public Works, 26110 Patras, Greece, [email protected] 2 University of Patras, Department of Geology, Laboratory of Engineering Geology, 26500 Patras, Greece, [email protected] Abstract In August 2007 Ilia Prefecture suffered one of the most devastating wildfires that have ever happened on European level. Approximately 870km2, mainly forest and agricultural land, were lost, more than 60 people were killed, hundreds were injured and many villages suffered ex- tensive damage. Heavy rainfall and human activities, favoured by the loss of vegetation and the overall susceptibility of geological formations in landsliding, induced the manifestation or re- activation of various scale landslide phenomena. In order to investigate and mitigate the prob- lem University of Patras was commissioned by the Region of Western Greece to undertake an Engineering Geological and Geotechnical investigation. Site investigation accomplished in seven municipalities focusing on several landslide events with serious socio-economic impact and as a result many small scale cases were identified. In -
Selido3 Part 1
ΔΕΛΤΙΟ ΤΗΣ ΕΛΛΗΝΙΚΗΣ ΓΕΩΛΟΓΙΚΗΣ ΕΤΑΙΡΙΑΣ Τόμος XLIII, Νο 3 BULLETIN OF THE GEOLOGICAL SOCIETY OF GREECE Volume XLIII, Νο 3 1 (3) ΕΙΚΟΝΑ ΕΞΩΦΥΛΛΟΥ - COVER PAGE Γενική άποψη της γέφυρας Ρίου-Αντιρρίου. Οι πυλώνες της γέφυρας διασκοπήθηκαν γεωφυ- σικά με χρήση ηχοβολιστή πλευρικής σάρωσης (EG&G 4100P και EG&G 272TD) με σκοπό την αποτύπωση του πυθμένα στην περιοχή του έργου, όσο και των βάθρων των πυλώνων. (Εργα- στήριο Θαλάσσιας Γεωλογίας & Φυσικής Ωκεανογραφίας, Πανεπιστήμιο Πατρών. Συλλογή και επεξεργασία: Δ.Χριστοδούλου, Η. Φακίρης). General view of the Rion-Antirion bridge, from a marine geophysical survey conducted by side scan sonar (EG&G 4100P and EG&G 272TD) in order to map the seafloor at the site of the construction (py- lons and piers) (Gallery of the Laboratory of Marine Geology and Physical Oceanography, University of Patras. Data acquisition and Processing: D. Christodoulou, E. Fakiris). ΔΕΛΤΙΟ ΤΗΣ ΕΛΛΗΝΙΚΗΣ ΓΕΩΛΟΓΙΚΗΣ ΕΤΑΙΡΙΑΣ Τόμος XLIII, Νο 3 BULLETIN OF THE GEOLOGICAL SOCIETY OF GREECE Volume XLIII, Νο 3 12o ΔΙΕΘΝΕΣ ΣΥΝΕΔΡΙΟ ΤΗΣ ΕΛΛΗΝΙΚΗΣ ΓΕΩΛΟΓΙΚΗΣ ΕΤΑΙΡΙΑΣ ΠΛΑΝHΤΗΣ ΓH: Γεωλογικές Διεργασίες και Βιώσιμη Ανάπτυξη 12th INTERNATIONAL CONGRESS OF THE GEOLOGICAL SOCIETY OF GREECE PLANET EARTH: Geological Processes and Sustainable Development ΠΑΤΡΑ / PATRAS 2010 ISSN 0438-9557 Copyright © από την Ελληνική Γεωλογική Εταιρία Copyright © by the Geological Society of Greece 12o ΔΙΕΘΝΕΣ ΣΥΝΕΔΡΙΟ ΤΗΣ ΕΛΛΗΝΙΚΗΣ ΓΕΩΛΟΓΙΚΗΣ ΕΤΑΙΡΙΑΣ ΠΛΑΝΗΤΗΣ ΓΗ: Γεωλογικές Διεργασίες και Βιώσιμη Ανάπτυξη Υπό την Αιγίδα του Υπουργείου Περιβάλλοντος, Ενέργειας και Κλιματικής Αλλαγής 12th INTERNATIONAL CONGRESS OF THE GEOLOGICAL SOCIETY OF GREECE PLANET EARTH: Geological Processes and Sustainable Development Under the Aegis of the Ministry of Environment, Energy and Climate Change ΠΡΑΚΤΙΚΑ / PROCEEDINGS ΕΠΙΜΕΛΕΙΑ ΕΚΔΟΣΗΣ EDITORS Γ. -

REDUCTION 2011-2014 Deliverable 5.2 Report on Collective Evaluation
REDUCTION 2011‐2014 Deliverable 5.2 Report on Collective Evaluation from Field Trials in Phase‐1 31 August 2014 D5.2 [Report on Collective Evaluation from Field Trials in Phase‐1] Public Document II D5.2 [Report on Collective Evaluation from Field Trials in Phase‐1] Project acronym: REDUCTION Project full title: Reducing Environmental Footprint based on Multi‐Modal Fleet management Systems for Eco‐Routing and Driver Behaviour Adaptation Work Package: WP5 Document title: Report on Collective Evaluation from Field Trials in Phase‐1 Version: 5.0 Official delivery date: 31/08/2014 Actual publication date: 31/08/2014 Type of document: Report Nature: Public Authors: Dimitrios Katsaros (UTH), Chrysi Laspidou (UTH), Stavroula Maglavera (UTH), Nikolaos Lemonas (UTH), Kristian Torp (AAU), Ove Andersen (AAU), Kyriacos Mouskos (CTL), Athanasios Lois (TrainOSE), Marcel Morssink (TRI) III D5.2 [Report on Collective Evaluation from Field Trials in Phase‐1] Approved by: REDUCTION consortium partners Version Date Sections Affected 0.1 27/12/2012 Initial empty template 1.0 25/01/2013 Updated by Aalborg, FlexDanmark 1.1 05/02/2013 Updated by TrainOSE 1.2 18/02/2013 Updated by Aalborg, FlexDanmark 1.3 21/02/2013 Updated by UTH 1.4 22/02/2013 Updated by CTL 2.1 23/02/2013 Updated to correct various issues 2.2 27/02/2013 Review comments processed 3.0 19/08/2013 Major updates by TRI and CTL 4.0 12/02/2014 Updated to reflect 2nd review comments 5.0 26/08/2014 Various changes and corrections IV D5.2 [Report on Collective Evaluation from Field Trials in Phase‐1] Executive Summary Field operational testing is widely recognized as an effective instrument to test new transport technologies and methodologies in the real world. -

Snowe: Iraq Must Solve Its Problems Politically Leadership 100 To
O C V ΓΡΑΦΕΙ ΤΗΝ ΙΣΤΟΡΙΑ Bringing the news ΤΟΥ ΕΛΛΗΝΙΣΜΟΥ to generations of ΑΠΟ ΤΟ 1915 The National Herald Greek Americans A WEEKLY GREEK AMERICAN PUBLICATION c v www.thenationalherald.com VOL. 10, ISSUE 521 October 6, 2007 $1.00 GREECE: 1.75 EURO Alex Spanos Snowe: Iraq Donates $1 Must Solve Million to Its Problems Fire Victims Politically Deeply Moved by Republican Senator Suffering Caused by Says U.S. Can’t Prop Destructive Flames Iraq Up Indefinitely By Theodore Kalmoukos Special to The National Herald By Evan C. Lambrou Special to The National Herald BOSTON – The family of California real estate magnate Alex Spanos NEW YORK – The Iraqi Government has donated $1 million to the Arch- has failed to achieve the necessary diocese’s Greek Fire Relief Fund, level of political reconciliation, the the Archdiocese announced late last main component for creating stabili- week. ty in Iraq, according to U.S. Senator When making his contribution to Olympia J. Snowe of Maine, so the the Fund, Mr. Spanos said he and U.S. needs to gradually reduce the his family were deeply moved by number of troops and eventually the pain and suffering inflicted by withdraw from Iraq. the destructive flames. U.S. forces can not guarantee “We watched with heavy hearts Iraq’s future security, she says, be- the catastrophic fires and the de- cause that ultimately has to come struction they left behind that af- from the Iraqis themselves. fected thousands of people, their In an interview with the National property and the beautiful country- TNH/COSTAS BEJ Herald this past Tuesday, October 2, side of Greece. -

Reference Evapotranspiration
International Journal of Geo-Information Article Reference Evapotranspiration (ETo) Methods Implemented as ArcMap Models with Remote-Sensed and Ground-Based Inputs, Examined along with MODIS ET, for Peloponnese, Greece Stavroula Dimitriadou and Konstantinos G. Nikolakopoulos * Department of Geology, University of Patras, 26504 Rion, Greece; [email protected] * Correspondence: [email protected]; Tel.: +30-261-099-759-2 Abstract: The present study develops ArcMap models to implement the following three methods: FAO-56 Penman–Monteith (FAO PM), Hargreaves–Samani (HS) and Hansen, with the former used as a reference. Moreover, three models implementing statistical indices (RMSD, MB, NMB) are also created. The purpose is threefold, as follows: to investigate the variability in the daily mean reference evapotranspiration (ETo) for the Decembers and Augusts during 2016–2019, over Pelopon- nese, Greece. Furthermore, to investigate the agreement between the methods’ ETo estimates, and examine the former along with MODIS ET (daily) averaged products. The study area is a complex Mediterranean area. Meteorological data from sixty-two stations under the National Observatory of Athens (NOA), and MODIS Terra LST products, have been employed. FAO PM is found sensitive to Citation: Dimitriadou, S.; wind speed and depicts interactions among climate parameters (T, evaporative demand and water Nikolakopoulos, K.G. Reference availability) in the frame of climate change. The years 2016–2019 are four of the warmest since the Evapotranspiration (ETo) Methods preindustrial era. Hargreaves–Samani’s estimations for the Decembers of 2016–2019 were almost Implemented as ArcMap Models with identical to MODIS ET, despite their different physical meaning. However, for the Augusts there are Remote-Sensed and Ground-Based considerable discrepancies between the methods’ and MODIS’s estimates, attributed to the higher Inputs, Examined along with MODIS evaporative demand in the summertime. -

Predicting Fish Species Diversity in Lotic Freshwaters of Greece 1 2 2 3 Eugene G
Virginia Journal of Science Volume 54, Number 3 & 4 Fall & Winter 2003 Predicting Fish Species Diversity in Lotic Freshwaters of Greece 1 2 2 3 Eugene G. Maurakis ' and David V. Grimes ' lSc1ence Museum of Virginia, 2500 W. Broad St., Richmond, VA 23220 USA 2Biology Dept. and School of Continuing Studies, University of Richmond, VA 23173 USA 3Environmental Division, VA Dept. of Transportation, 1200 E Broad St., Richmond, VA 23219 Keywords: freshwater fish diversity, Greece ABSTRACT Objectives were to test the hypothesis that stream order and stream width alone account for species diversity in drainages of Greece, and to create a mathematical model that predicts fish diversity in small and medium sized freshwater streams in the southern Balkan Peninsula in accord with the stream classification system proposed by the European Environmental Agency (EEA). Thirty-seven species of fishes in 12 families (Petromyzontidae, An guillidae, Cyprinidae, Cobitidae, Balitoridae, Mugilidae, Salmonidae, Peo ciliidae, Gasterosteidae, Moronidae, Centrarchidae, and Blenniidae) were collected in five stream orders (1-5) from 19 river drainages in Greece in 1993 and from 2000-2002. Numbers of species were significantly correlated with stream order ( +), width ( +), and depth ( +), and elevation (-). Results of stepwise regression indicated that stream order, elevation, stream depth, and river km were significant factors associated with ichthyofaunal diversity, an~ were used to create a regression model to predict species diversity (up to 5 order streams). We conclude that geo-specific factors (i.e., small, isolated drainages with limited water budgets, geological history, dry climate, and low annual rainfall) should be included in the EEA monitoring design for lotic waters in harsh environments of southern Mediterranean countries as these features differ from those ofcentral, eastern, and northern European countries with larger watersheds. -

December2019 Erasmus 2Nd Junior High School of Amaliada 2019-2020
2nd Junior High School of Amaliada ERASMUS+ PROGRAMME KA 2 STRATEGIC PARTNERSHIP “European Schools Go Green” 2017 - 2020 DECEMBER 2019 PRESS RELEASE With intensive preparations, activities, work, hospitality and endless creativity we are continuing enthousiasticly the third and final year of the European Erasmus + Programme / KA2 Key Action / "Collaboration for Innovation and Exchange of Good Practices" 2017-2020 entitled "European Schools Go Green" 2017-2020 at the 2nd Junior High School of Amaliada! After two exceptionally creative years, we reached the third and final year of this European collaboration, having already exchanged visits and hosted students and teachers from both schools abroad with success and excellent results. Our students and teachers have already also traveled and have been warmly hosted in Genoa, Italy, and in Kassel, Germany, returning with life experiences and spiritual and mental resources. A collaboration with great dissemination of our work and a particularly warm response from the local community, the school and family environment of the students, the numerous collaborators and local authoritiew and many institutions we are working with, with publications and extensive articles in print and digital Greek, Italian and German Press, a tribute from local television, the website of the Directorate of Education of Western Greece which posted articles about our collaboration and our digital products on the "best practices" website and more. The pedagogical team of the 2nd Junior High School of Amaliada: From left are Teachers: Mr Haris Spiropoulos, Mrs Efi Karvouniaris, Mr Grigoris Vassilopoulos, Principal Mr Antonis Gounaris, Mrs Maria Tzavara, Mrs Eleni Charda, Mrs Antonia Rambavila, Mrs Stavroula Salvanou, Mrs Katerina Andreou.