Surveillance for Hepatocellular Carcinoma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oligometastatic Adenocarcinoma of the Esophagus: Current Understanding, Diagnosis, and Therapeutic Strategies
cancers Review Oligometastatic Adenocarcinoma of the Esophagus: Current Understanding, Diagnosis, and Therapeutic Strategies Michael P. Rogers 1 , Anthony J. DeSantis 1 and Christopher G. DuCoin 2,* 1 Department of Surgery, Morsani College of Medicine, University of South Florida, Tampa, FL 33606, USA; [email protected] (M.P.R.); [email protected] (A.J.D.) 2 Department of Surgery, Division of Gastrointestinal Surgery, Morsani College of Medicine, University of South Florida, Tampa, FL 33606, USA * Correspondence: [email protected] Simple Summary: The diagnosis and management of oligometastatic esophageal adenocarcinoma remains nuanced. Early diagnosis may allow for prompt intervention and, ideally, prolonged patient survival. As recent and emerging trials shed new light on this topic, we sought to identify the current understanding and treatment recommendations for oligometastatic disease by performing a thorough review of the available literature. Abstract: Esophageal adenocarcinoma is an aggressive cancer of increasing incidence and is associ- ated with poor prognosis. The early recognition of synchronous and metachronous oligometastasis in esophageal adenocarcinoma may allow for prompt intervention and potentially improved survival. However, curative approaches to oligometastatic esophageal disease remain unproven and may represent an area of emerging divergence of opinion for surgical and medical oncologists. We sought to identify the current understanding and evidence for management of oligometastatic esophageal Citation: Rogers, M.P.; -

Diagnosis Accuracy and Prognostic Significance of the Dickkopf-1
Journal of Cancer 2020, Vol. 11 7091 Ivyspring International Publisher Journal of Cancer 2020; 11(24): 7091-7100. doi: 10.7150/jca.49970 Review Diagnosis Accuracy and Prognostic Significance of the Dickkopf-1 Protein in Gastrointestinal Carcinomas: Systematic Review and Network Meta-analysis Xiaowen Jiang1#, Fuhai Hui1#, Xiaochun Qin1, Yuting Wu1, Haihan Liu1, Jing Gao2, Xiang Li2, Yali Xu2, Yingshi Zhang1 1. Department of Life Science and Biochemistry, Shenyang Pharmaceutical University, Shenyang, 110016, China. 2. Department of Pharmacy, Shenyang Pharmaceutical University, Shenyang, 110016, China. #Xiaowen Jiang and Fuhai Hui contributed equally to this article Corresponding author: Yingshi Zhang, Department of Life Science and Biochemistry, Shenyang Pharmaceutical University, No. 103 Wenhua Road, Shenyang, 110016, China. E-mail: [email protected], [email protected] (Y. Zhang) © The author(s). This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See http://ivyspring.com/terms for full terms and conditions. Received: 2020.06.26; Accepted: 2020.10.06; Published: 2020.10.18 Abstract Objective: To evaluate the diagnosis accuracy and prognostic significance of bio-marker dickkopf-1(DKK-1) protein in GIC, and also sub-type of hepatocellular carcinoma (HCC), pancreas carcinomas (PC), oesophageal carcinoma (EPC) and Adenocarcinoma of esophago-gastric junction (AEGJ), etc. Methods: Electronic databases were searched from inception to May 2020. Patients were diagnosed with gastrointestinal carcinomas, and provided data on the correlation between high and low DKK-1 expression and diagnosis or prognosis. Results: Forty-three publications involving 9318 participants were included in the network meta-analysis, with 31 of them providing data for diagnosis value and 18 records were eligible for providing prognosis value of DKK-1. -
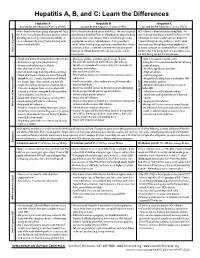
Hepatitis A, B, and C: Learn the Differences
Hepatitis A, B, and C: Learn the Differences Hepatitis A Hepatitis B Hepatitis C caused by the hepatitis A virus (HAV) caused by the hepatitis B virus (HBV) caused by the hepatitis C virus (HCV) HAV is found in the feces (poop) of people with hepa- HBV is found in blood and certain body fluids. The virus is spread HCV is found in blood and certain body fluids. The titis A and is usually spread by close personal contact when blood or body fluid from an infected person enters the body virus is spread when blood or body fluid from an HCV- (including sex or living in the same household). It of a person who is not immune. HBV is spread through having infected person enters another person’s body. HCV can also be spread by eating food or drinking water unprotected sex with an infected person, sharing needles or is spread through sharing needles or “works” when contaminated with HAV. “works” when shooting drugs, exposure to needlesticks or sharps shooting drugs, through exposure to needlesticks on the job, or from an infected mother to her baby during birth. or sharps on the job, or sometimes from an infected How is it spread? Exposure to infected blood in ANY situation can be a risk for mother to her baby during birth. It is possible to trans- transmission. mit HCV during sex, but it is not common. • People who wish to be protected from HAV infection • All infants, children, and teens ages 0 through 18 years There is no vaccine to prevent HCV. -

Active Peptic Ulcer Disease in Patients with Hepatitis C Virus-Related Cirrhosis: the Role of Helicobacter Pylori Infection and Portal Hypertensive Gastropathy
dore.qxd 7/19/2004 11:24 AM Page 521 View metadata, citation and similar papers at core.ac.uk ORIGINAL ARTICLE brought to you by CORE provided by Crossref Active peptic ulcer disease in patients with hepatitis C virus-related cirrhosis: The role of Helicobacter pylori infection and portal hypertensive gastropathy Maria Pina Dore MD PhD, Daniela Mura MD, Stefania Deledda MD, Emmanouil Maragkoudakis MD, Antonella Pironti MD, Giuseppe Realdi MD MP Dore, D Mura, S Deledda, E Maragkoudakis, Ulcère gastroduodénal évolutif chez les A Pironti, G Realdi. Active peptic ulcer disease in patients patients atteints de cirrhose liée au HCV : Le with hepatitis C virus-related cirrhosis: The role of Helicobacter pylori infection and portal hypertensive rôle de l’infection à Helicobacter pylori et de la gastropathy. Can J Gastroenterol 2004;18(8):521-524. gastropathie liée à l’hypertension portale BACKGROUND & AIM: The relationship between Helicobacter HISTORIQUE ET BUT : Le lien entre l’infection à Helicobacter pylori pylori infection and peptic ulcer disease in cirrhosis remains contro- et l’ulcère gastroduodénal dans la cirrhose reste controversé. Le but de la versial. The purpose of the present study was to investigate the role of présente étude est de vérifier le rôle de l’infection à H. pylori et de la gas- H pylori infection and portal hypertension gastropathy in the preva- tropathie liée à l’hypertension portale dans la prévalence de l’ulcère gas- lence of active peptic ulcer among dyspeptic patients with compen- troduodénal évolutif chez les patients dyspeptiques souffrant d’une sated hepatitis C virus (HCV)-related cirrhosis. -

Understanding Human Astrovirus from Pathogenesis to Treatment
University of Tennessee Health Science Center UTHSC Digital Commons Theses and Dissertations (ETD) College of Graduate Health Sciences 6-2020 Understanding Human Astrovirus from Pathogenesis to Treatment Virginia Hargest University of Tennessee Health Science Center Follow this and additional works at: https://dc.uthsc.edu/dissertations Part of the Diseases Commons, Medical Sciences Commons, and the Viruses Commons Recommended Citation Hargest, Virginia (0000-0003-3883-1232), "Understanding Human Astrovirus from Pathogenesis to Treatment" (2020). Theses and Dissertations (ETD). Paper 523. http://dx.doi.org/10.21007/ etd.cghs.2020.0507. This Dissertation is brought to you for free and open access by the College of Graduate Health Sciences at UTHSC Digital Commons. It has been accepted for inclusion in Theses and Dissertations (ETD) by an authorized administrator of UTHSC Digital Commons. For more information, please contact [email protected]. Understanding Human Astrovirus from Pathogenesis to Treatment Abstract While human astroviruses (HAstV) were discovered nearly 45 years ago, these small positive-sense RNA viruses remain critically understudied. These studies provide fundamental new research on astrovirus pathogenesis and disruption of the gut epithelium by induction of epithelial-mesenchymal transition (EMT) following astrovirus infection. Here we characterize HAstV-induced EMT as an upregulation of SNAI1 and VIM with a down regulation of CDH1 and OCLN, loss of cell-cell junctions most notably at 18 hours post-infection (hpi), and loss of cellular polarity by 24 hpi. While active transforming growth factor- (TGF-) increases during HAstV infection, inhibition of TGF- signaling does not hinder EMT induction. However, HAstV-induced EMT does require active viral replication. -

Hepatitis C 2005 Clinical Guidelines Summary of The: New York State Department of Health Clinical Guidelines for the Medical Management of Hepatitis C
Hepatitis C 2005 Clinical Guidelines Summary of the: New York State Department of Health Clinical Guidelines for the Medical Management of Hepatitis C Inside: Key Features of Viral Hepatitis A,B and C 1 Natural Course of HCV Infection 2 Persons at Risk for HCV Infection 3 Sources of HCV Infection 4 Counseling Prior To Testing 5 Screening for HCV Algorithm 6 Laboratory Testing for HCV 7 Interpretation of HCV Test Results 8 Post Exposure Screening for HCV 9 Counseling After Testing 10 Treating HCV Patients 11 Medical Management of HCV Positive Patients 12 References and Internet Resources 13 Hepatitis C Virus The Hidden Epidemic The Burden of HCV • 3 million Americans are chronically infected with the • 8-10,000 deaths a year are caused by HCV. Hepatitis C virus (HCV). • HCV is the leading cause for liver transplants and • 342,000 New Yorkers are estimated to be infected chronic liver disease. with HCV. • HCV deaths will increase four-fold to 38,000, by • A majority of the people infected with HCV do not the year 2010. know they have it. • Years of life lost to Hepatitis C (2001-2019) • Thousands of people go undetected each year—due 3.1 million years to inadequate risk assessment, under-screening and confusion about the use of diagnostic tests. • Cost of premature disability and death (2010-2109) $75.5 billion Hepatitis C Virus • Direct medical costs in absentee losses due to Hepatitis C $750 million/ year • HCV is a blood-borne disease transmitted by • Total medical expenditures for persons with HCV blood-to-blood contact. -

Hepatitis C Virus Infection and Human Pancreatic ß-Cell Dysfunction
Pathophysiology/Complications BRIEF REPORT Hepatitis C Virus Infection and Human Pancreatic -Cell Dysfunction 1 1 MATILDE MASINI, MD SILVIA DEL GUERRA, PHD showing the characteristic insulin gran- 2 1 DANIELA CAMPANI, MD MARCO BUGLIANI, PHD ules and normally preserved mitochon- 3 1 UGO BOGGI, MD SCILLA TORRI, PHD  2 1 dria. In -cells from HCV-positive MICHELE MENICAGLI, MD STEFANO DEL PRATO, MD 1 3 pancreases, the presence of virus-like par- NICOLA FUNEL, MD FRANCO MOSCA, MD 1 4 ticles was observed, mainly close to the MARIA POLLERA, MD FRANCO FILIPPONI, MD 1 1 membranes of Golgi apparatus, which, in ROBERTO LUPI, PHD PIERO MARCHETTI, MD, PHD turn, appeared hyperplastic and dilated (Fig. 1D). The mitochondria appeared round-shaped with dispersed matrix and fragmented cristae (Fig. 1D). Additional Ϯ 2 any patients with chronic hepati- and 2 women, BMI 25.8 1.6 kg/m ) -cell changes were observed at the Ϯ tis C virus (HCV) develop type 2 and 10 HCV-negative (age 67 9 years, level of rough endoplasmic reticulum, Ϯ diabetes (1). This prevalence is 6 men and 4 women, BMI 26.8 2.0 which showed long and dilated tubular M 2 much higher than that observed in the kg/m ) donors were harvested and stud- membranes, with numerous electron- general population and in patients with ied with the approval of our local ethics dense ribosomes bound to the latter (not other chronic liver diseases such as hepa- committee. Histological studies were shown). These morphological changes titis B virus, alcoholic liver disease, and performed by immunohistochemistry were accompanied by reduced in vitro primary biliary cirrhosis. -

Synchronous Pancreatic Ductal Adenocarcinoma and Hepatocellular Carcinoma: Report of a Case and Review of the Literature JENNY Z
ANTICANCER RESEARCH 38 : 3009-3012 (2018) doi:10.21873/anticanres.12554 Synchronous Pancreatic Ductal Adenocarcinoma and Hepatocellular Carcinoma: Report of a Case and Review of the Literature JENNY Z. LAI 1,2 , YIHUA ZHOU 3 and DENGFENG CAO 2 1University College, Washington University in St. Louis, St. Louis, MO, U.S.A.; 2Department of Pathology and Immunology, Washington University in Saint Louis School of Medicine, St. Louis, MO, U.S.A.; 3Department of Radiology, University of Pittsburgh School of Medicine, Pittsburgh, PA, U.S.A. Abstract. Two or more histologically distinct malignancies adenocarcinoma (PDAC) is one of the fatal cancers with diagnosed during the same hospital admission are short-term survival rates. The case fatality rate for the uncommon, but they do exist. Cases with synchronous disease is approximately 97% and has declined slightly (from primary pancreatic ductal adenocarcinoma and 99% to 97%) in the last 12 years (3). To date, the causes of hepatocellular carcinoma are rarely seen. This is a case pancreatic cancer are not thoroughly understood, though report of a 56 years old Caucasian female with the chief certain risk factors such as smoking, obesity, lifestyle, complaint of jaundice over a duration of 10 days. CT diabetes mellitus, alcohol, dietary factors and chronic imaging findings revealed a 3.5 cm ill-defined pancreatic pancreatitis have been proposed. Hepatocellular carcinoma head mass and a 1.5 cm liver mass in the segment 5. EUS- (HCC) is the second leading cause and the fastest rising FNA cytology showed pancreatic head ductal cause of cancer-related death worldwide (4). One of the most adenocarcinoma (PDAC). -

Helicobacter Hepaticus Model of Infection: the Human Hepatocellular Carcinoma Controversy
Review articles Helicobacter hepaticus model of infection: the human hepatocellular carcinoma controversy Yessica Agudelo Zapata, MD,1 Rodrigo Castaño Llano, MD,2 Mauricio Corredor, PhD.3 1 Medical Doctor and General Surgeon in the Abstract Gastrohepatology Group at Universidad de Antioquia in Medellin, Colombia The discovery of Helicobacter 30 years ago by Marshall and Warren completely changed thought about peptic 2 Gastrointestinal Surgeon and Endoscopist in the and duodenal ulcers. The previous paradigm posited the impossibility of the survival of microorganisms in the Gastrohepatology Group at the Universidad de stomach’s low pH environment and that, if any microorganisms survived, they would stay in the duodenum Antioquia in Medellin, Colombia 3 Institute Professor of Biology in the Faculty of or elsewhere in the intestine. Today the role of H. pylori in carcinogenesis is indisputable, but little is known Natural Sciences and the GEBIOMIC Group the about other emerging species of the genus Helicobacter in humans. Helicobacter hepaticus is one of these Gastroenterology Group at the Universidad de species that has been studied most, after H. pylori. We now know about their microbiological, genetic and Antioquia in Medellin, Colombia pathogenic relationships with HCC in murine and human infections. This review aims to show the medical and ......................................... scientifi c community the existence of new species of Helicobacter that have pathogenic potential in humans, Received: 07-05-13 thus encouraging research. Accepted: 27-08-13 Keywords Helicobacter hepaticus, Helicobacter pylori, Helicobacter spp., hepatocellular carcinoma. INTRODUCTION bacterium can infect animals including mice, dogs and ger- bils which could lead to a proposal to use H. -

Vaccinations for Adults with Chronic Liver Disease Or Infection
Vaccinations for Adults with Chronic Liver Disease or Infection This table shows which vaccinations you should have to protect your health if you have chronic hepatitis B or C infection or chronic liver disease (e.g., cirrhosis). Make sure you and your healthcare provider keep your vaccinations up to date. Vaccine Do you need it? Hepatitis A Yes! Your chronic liver disease or infection puts you at risk for serious complications if you get infected with the (HepA) hepatitis A virus. If you’ve never been vaccinated against hepatitis A, you need 2 doses of this vaccine, usually spaced 6–18 months apart. Hepatitis B Yes! If you already have chronic hepatitis B infection, you won’t need hepatitis B vaccine. However, if you have (HepB) hepatitis C or other causes of chronic liver disease, you do need hepatitis B vaccine. The vaccine is given in 2 or 3 doses, depending on the brand. Ask your healthcare provider if you need screening blood tests for hepatitis B. Hib (Haemophilus Maybe. Some adults with certain high-risk conditions, for example, lack of a functioning spleen, need vaccination influenzae type b) with Hib. Talk to your healthcare provider to find out if you need this vaccine. Human Yes! You should get this vaccine if you are age 26 years or younger. Adults age 27 through 45 may also be vacci- papillomavirus nated against HPV after a discussion with their healthcare provider. The vaccine is usually given in 3 doses over a (HPV) 6-month period. Influenza Yes! You need a dose every fall (or winter) for your protection and for the protection of others around you. -

Dyspepsia in Cirrhotic Hepatitis C Patients
Journal of Rawalpindi Medical College (JRMC); 2017;21(4): 321-324 Original Article Dyspepsia in Cirrhotic Hepatitis C Patients Maryam Jalil1, Faiza Aslam 2, Talal Khurshid Bhatti 3 , Muhammad Umar 1 , Hamamatul Bushra Khaar 1 1Woman Medical Officer,THQ Hospital ,Fateh Jang;2. Research Coordinator at Research Unit, Rawalpindi Medical University; 3. Medical officer, THQ Hospital,Lalamusa. Abstract positive individuals and patients with liver cirrhosis.2 Indigestion, also known as dyspepsia, is a condition of Background:To determine the frequency of impaired digestion characterized by upper abdominal patients with dyspepsia, its patterns of presentation fullness, heartburn, nausea, belching, upper and causes along with their associations with gender abdominal pain, regurgitation, or water brash (bitter and age, amongst HCV cirrhotic patients presenting tasting liquid coming up into back of throat).3About to a tertiary care health facility of Rawalpindi. 30-40% of all adults experience symptoms of Methods: In this cross sectional study 207 HCV dyspepsia but organic cause is found in only few cases cirrhotic patients,above 25 years of age irrespective who seek medical care. The remaining cases are of gender, were included. Patients receiving labelled as having functional dyspepsia.4 Different prolonged treatment of acid suppression prior to causes of dyspepsia are gastroesophageal reflux hospitalization were excluded. After taking history disease (GERD), peptic ulcer, gastrointestinal cancers, and performing thorough physical examination, gastritis, hiatal hernia, gastroparesis, congestive routine laboratory investigations, abdominal gastropathy, cholelithiasis, food or drug intolerance ultrasonography and endoscopies were performed to and other diseases of gastrointestinal tract, liver, determine the cause of dyspepsia. pancreas and other systems.5-7 Results:Amongst 207 HCV cirrhotic patients 146 World Health Organization Statistics 2008 lists (70.5%) were presented with dyspepsia. -

Non-Alcoholic Fatty Liver Disease Information for Patients
April 2021 | www.hepatitis.va.gov Non-Alcoholic Fatty Liver Disease Information for Patients What is Non-Alcoholic Fatty Liver Disease? Losing more than 10% of your body weight can improve liver inflammation and scarring. Make a weight loss plan Non-alcoholic fatty liver disease or NAFLD is when fat is with your provider— and exercise to keep weight off. increased in the liver and there is not a clear cause such as excessive alcohol use. The fat deposits can cause liver damage. Exercise NAFLD is divided into two types: simple fatty liver and non- Start small, with a 5-10 minute brisk walk for example, alcoholic steatohepatitis (NASH). Most people with NAFLD and gradually build up. Aim for 30 minutes of moderate have simple fatty liver, however 25-30% have NASH. With intensity exercise on most days of the week (150 minutes/ NASH, there is inflammation and scarring of the liver. A small week). The MOVE! Program is a free VA program to help number of people will develop significant scarring in their lose weight and keep it off. liver, called cirrhosis. Avoid Alcohol People with NAFLD often have one or more features of Minimize alcohol as much as possible. If you do drink, do metabolic syndrome: obesity, high blood pressure, low HDL not drink more than 1-2 drinks a day. Patients with cirrhosis cholesterol, insulin resistance or diabetes. of the liver should not drink alcohol at all. NAFLD increases the risk for diabetes, cardiovascular disease, Treat high blood sugar and high cholesterol and kidney disease. Ask your provider if you have high blood sugar or high Most people feel fine and have no symptoms.