Xanthomonas Campestris Pv. Coriandri on Coriander
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Essential Oil Proudly South African & Foreign Formulations Presented by Professor Aubrey Parsons 2019
Essential oil Proudly South African & Foreign Formulations Presented by Professor Aubrey Parsons 2019 Photo by Itineranttrader / Public domain • Angelica (Angelica archangelica) • Aniseed (Illicium Verum) • Basil (Ocimum basilicum) • Bay (Pimenta racemose) • Benzoin resinoid (Styrax benzoin) • Bergamot (Citrus bergamia) Essential Oils • Black Pepper (Piper nigrum) • Cajuput (Melaleuca cajiputi) • Camphor (Cinnamomum camphora) • Cardamom (Eletarria cardamomum) • Carraway (Carum carvi) • Carrot Seed (Daucus carota • Cedarwood Atlas (Cedrus atlantica) • Chamomile Blue Egyptian (Matricaria recutita) • Chamomile Wild Maroc (Ormensis multicaulis) • Chamomile Roman (Anthemis nobilis) • Cinnamon (Cinnamon zaylanicum) • Citronella (Cymbopogon nardus) • Clary Sage (Salvia sclarea) Essential Oils • Clove Leaf (Eugenia caryophyllata) • Coriander (Coriandrum sativum) • Cummin (Cuminum cyminum) • Cypress (Cupressa sempervirens) • Eucalyptus (Eucalyptus globulus) • Fennel (Foeniculum vulgare) • Frankincense Olibanum (Boswellia thurifera) • Garlic (Allium sativum) • Geranium (Pelargonium graveolens) • Ginger (Zingiber officinale) • Grapefruit (Citrus paradise) • Ho Leaf (Cinnamomum camphora • Hyssop (Hyssopus officinalis) Essential Oils • Jasmine Absolute (Jasmine officinalis) • Juniper (Juniperus communis) • Lavender (Lavandula angustifolium) • Lemon (Citrus limonum) • Lemongrass (Cymbopogon citratus) • Lime (Citrus aurrantifolia) • Mandarin (Citrus reticulata bianco) • Marjoram Cultivated (Marjorama hortensis) • Myrrh (Commiphora Myrrha) • Neroli (Citrus -

Food Recommendations Lovage Perennial Edible Herb Originally
Herbs-Who Are You?/ Written by Tome Shaaltiel, presented by Joan Plisko Herb ID pointers Medicinal value Fun fact! Food recommendations Lovage Perennial Some people Likely In foods and edible herb take lovage by unsafe to beverages, lovage originally from mouth as consume is used for Mediterranean "irrigation during flavoring. region, In the therapy" for pain pregnancy. carrot family, and swelling leaves look (inflammation) of like celery, the lower urinary grows up to tract, for 6ft tall, foliage preventing of is in an kidney stones, umbrella form and to increase with yellow the flow of urine flowers, roots during urinary are greyish tract infections. pink. But there is no good scientific research to support the use of lovage for these or other conditions. The oil can cause irritation to skin in a pure form. Thyme Perennial, essential oils in Can help Lemon thyme is woody stems thyme are you’re your great in a lemon with tiny green packed with anti- snoring cur or lemon pie. leaves on septic, anti-viral, problems! French or Italian opposite sides anti-rheumatic, Thyme is great in of the stem, anti-parasitic and quiches and purple or anti- lasagna. white flower fungal properties, depends on great immune the variety, system booster. grow in a The oil can cause bush. irritation to skin in a pure form. Comfrey Perennial, big If chewing it up Comfrey has Don't eat please! furry leaves, with your teeth deep roots that can stick or with a grinder that reach to clothes, all into a paste or the aquifer grow from one wrapping the bringing stem, purple leaves around energy and flowers, not fractures, water from edible. -

2018-01-26 Langual Proposal from Foodex2 – Plants in Facet B
2018-01-26 LanguaL proposal from FoodEx2 – plants in facet B The following are proposals to update LanguaL Facet B, after having indexed EFSA FoodEx2 Exposure hierarchy 20170919. To these, I have added previously-submitted 2017 proposals based on GS1 that have not (yet) been included in LanguaL facet B. GS1 terms and FoodEx2 terms in the following tables are just given to indicate the origin of the proposal. Comments are given in red. First, some simple additions of terms to the SYNONYM field, to make it easier to find descriptors in the LanguaL Food Product Indexer: descriptor synonyms FoodEx2 term FoodEx2 def WORMWOOD [B3433] Add SYN: artemisia vulgaris LITTLE RADISH [B2960] Add SYN: raphanus sativus BLACK RADISH [B2959] Add SYN: raphanus sativus niger PARSNIP [B1483] Add SYN: pastinaca sativa ARRACACHA [B3439] Add SYN: arracacia xanthorrhiza CHAYOTE [B1730] Add SYN: GS1 10006356 - Squash Squash, Choko, grown from Sechium edule (Choko) choko NEW ZEALAND SPINACH Add SYN: GS1 10006427 - New- Tetragonia tetragonoides Zealand Spinach [B1732] tetragonia tetragonoides JAPANESE MILLET Add : barnyard millet; A000Z Barnyard millet Echinochloa esculenta (A. Braun) H. Scholz, Barnyard millet or Japanese Millet. [B4320] echinochloa esculenta INDIAN LONG PEPPER Add SYN! A019B Long pepper fruit Piper longum [B2956] piper longum EUROPEAN ELDER Modify SYN: [B1403] sambucus spp. (which refers to broader term) Should be sambucus nigra DOG ROSE [B2961] ADD SYN: rosa canina LOOSE LEAF LETTUCE Add SYN: [B2087] lactusa sativa L. var. crispa LOLLO ROSSO [B2088] Add SYN: GS1 10006425 - Lollo Lactuca sativa L. var. crispa Rosso red coral lettuce JAVA APPLE [B3395] Add syn! syzygium samarangense Some existing descriptors would also greatly benefit from updated AI (and synonyms): FoodEx2 FoodEx2 def descriptor AI synonyms term ENDIVE [B1314] Add to AI: A00LD Escaroles There are two main varieties of cultivated C. -

Harvesting Herbs
222 N Havana Spokane WA 99202 (509) 477-2181 e-mail: [email protected] http://extension.wsu.edu/spokane/master-gardener-program/home-lawn-and-garden/ C159 HARVESTING HERBS Herb plants (the ‘h’ is pronounced in England, generally silent in America) are flowering plants whose above-ground stems do not become woody. The plants commonly referred to as ‘herbs’ are valued for their medicinal, decorative properties, culinary flavors, and aromatic scents. When you look at the entire spectrum of herbs available, different parts of individual plants - leaves, flowers, seeds and roots – can be used for different purposes: Plant leaves or foliage are commonly used for flavoring food. Many seeds have highly individual flavors. Some stems are important for flavoring. Flowers tend to be used for dyes and perfumes. Roots are used as vegetables or as medicinal constituents. Sometimes the entire plant is used. Parts of an herb plant, other than the leaves, that are used for food flavoring are called ‘spices.’ Harvest time for herbs is decided by the individual plant. Most aromatic herbs are ready just as buds open into full blossom. The plants then contain the most volatile oils, which provide the greatest amount of fragrance and flavor. There are exceptions to this rule. Sage leaves should be picked when buds first appear. Hyssop, lavender and thyme should be picked when in full bloom. Many herbs are ready to flower and therefore harvest in mid-summer. Throughout the growing season, pick healthy leaves (not those yellow or brown with age) to use whole or chopped in culinary dishes. -
Herbs Such As Spearmint
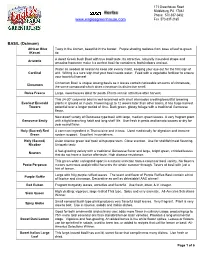
171 Greenhouse Road Middleburg, PA 17842 Phone: 570-837-0432 www.englesgreenhouse.com Fax 570-837-2165 BASIL (Ocimum) African Blue Tasty in the kitchen, beautiful in the border. Purple shading radiates from base of leaf to green (Kasar) tip. A dwarf Greek bush Basil with true basil taste. Its attractive, naturally mounded shape and Aristotle amazing fragrance make it a perfect basil for containers, both indoors and out. Water as needed all season to keep soil evenly moist, keeping your eye out for the first sign of Cardinal wilt. Wilting is a sure sign that your basil needs water. Feed with a vegetable fertilizer to ensure your bountiful harvest Cinnamon Basil is unique among basils as it leaves contain noticeable amounts of cinnamate, Cinnamon the same compound which gives cinnamon its distinctive smell. Dolce Fresca Large, sweet leaves ideal for pesto. Plants remain attractive after harvest. This 24-30” columnar basil is well-branched with short internodes creating beautiful towering Everleaf Emerald plants in ground or in pots. Flowering up to 12 weeks later than other basils, it has huge harvest Towers potential over a longer period of time. Dark green, glossy foliage with a traditional Genovese flavor. New dwarf variety of Genovese type basil with large, medium green leaves. A very fragrant plant Genovese Emily with a tight branching habit and long shelf life. Use fresh in pesto and tomato sauces or dry for year round flavor. Holy (Sacred) Red A common ingredient in Thai cuisine and in teas. Used medicinally for digestion and immune Green system support. -

Herbs, Spices and Essential Oils
Printed in Austria V.05-91153—March 2006—300 Herbs, spices and essential oils Post-harvest operations in developing countries UNITED NATIONS INDUSTRIAL DEVELOPMENT ORGANIZATION Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria Telephone: (+43-1) 26026-0, Fax: (+43-1) 26926-69 UNITED NATIONS FOOD AND AGRICULTURE E-mail: [email protected], Internet: http://www.unido.org INDUSTRIAL DEVELOPMENT ORGANIZATION OF THE ORGANIZATION UNITED NATIONS © UNIDO and FAO 2005 — First published 2005 All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of material in this information product for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission should be addressed to: - the Director, Agro-Industries and Sectoral Support Branch, UNIDO, Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria or by e-mail to [email protected] - the Chief, Publishing Management Service, Information Division, FAO, Viale delle Terme di Caracalla, 00100 Rome, Italy or by e-mail to [email protected] The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the United Nations Industrial Development Organization or of the Food and Agriculture Organization of the United Nations concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Spice Basics
SSpicepice BasicsBasics AAllspicellspice Allspice has a pleasantly warm, fragrant aroma. The name refl ects the pungent taste, which resembles a peppery compound of cloves, cinnamon and nutmeg or mace. Good with eggplant, most fruit, pumpkins and other squashes, sweet potatoes and other root vegetables. Combines well with chili, cloves, coriander, garlic, ginger, mace, mustard, pepper, rosemary and thyme. AAnisenise The aroma and taste of the seeds are sweet, licorice like, warm, and fruity, but Indian anise can have the same fragrant, sweet, licorice notes, with mild peppery undertones. The seeds are more subtly fl avored than fennel or star anise. Good with apples, chestnuts, fi gs, fi sh and seafood, nuts, pumpkin and root vegetables. Combines well with allspice, cardamom, cinnamon, cloves, cumin, fennel, garlic, nutmeg, pepper and star anise. BBasilasil Sweet basil has a complex sweet, spicy aroma with notes of clove and anise. The fl avor is warming, peppery and clove-like with underlying mint and anise tones. Essential to pesto and pistou. Good with corn, cream cheese, eggplant, eggs, lemon, mozzarella, cheese, olives, pasta, peas, pizza, potatoes, rice, tomatoes, white beans and zucchini. Combines well with capers, chives, cilantro, garlic, marjoram, oregano, mint, parsley, rosemary and thyme. BBayay LLeafeaf Bay has a sweet, balsamic aroma with notes of nutmeg and camphor and a cooling astringency. Fresh leaves are slightly bitter, but the bitterness fades if you keep them for a day or two. Fully dried leaves have a potent fl avor and are best when dried only recently. Good with beef, chestnuts, chicken, citrus fruits, fi sh, game, lamb, lentils, rice, tomatoes, white beans. -

Professional Report
1 Starozagorska street, floor 3, office 24-23 6100 Kazanlak, BULGARIA tel./ +359 431 623 99, fax/ +359 431 81231 office mobile: +359 899 98 77 88 PRICE LIST OF AVAILABLE ESSENTIAL OILS, ABSOLUTES, CONCRETES AND FLORAL WATER - ORGANIC AND CONVENTIONAL QUALITY BOTANICAL NAME ENGLISH NAME AVAILABLE EXW PRICE QUANTITY, KG PER KG IN EUR ESSENTIAL OILS Oleum Rosa Damascena Rose Otto 10.00 7200.00 Oleum Levistici Lovage life 11.00 700.00 Oleum Achilleae Millefolii “Proa” Yarrow 1.4 690.00 Oleum Achilleae Millefolii “Proa” Yarrow 18.00 660.00 Oleum Anthemis Nobilis Chamomilla Romana - NEW CROP Oleum Angelicae Archangelicae Angelica seeds 2.8 800.00 Oleum Melissae Officinalis Lemon balm 1.4 1800.00 Lavandula Angustifolia Mill. (Lavandula vera DC) * Lavender - NEW CROP Lavandula Angustifolia Mill. (Lavandula vera DC) Lavender 180.00 74.00 Hyssopus Officinalis L. Hyssop 1.00 120.00 Oleum Pini Sylvestris Pine 50.00 55.00 Oleum Juniperi Berry Juniper 150.00 130.00 Oleum Juniperi Communis Virgae Juniper, Needles - NEW CROP Oleum Geranium Macrorizum Zadravetz 2.00 1300.00 Oleum Hyperici Perforati* Saint John’s Word 3.00 910.00 Oleum Tanacetii parthenium Feverfew 4.7 104.00 Oleum Thymi sp. Diversae Thymus 20.00 325.00 Oleum Pinus Mugo Pine Mugo 5.00 60.00 Oleum Artemisia Annua Wormwood 100.00 90.00 Oleum Juniperus Oxicedrus fructus Prickly Juniper - NEW CROP Oleum Abies Alba Silver Fir - NEW CROP e-mail: asenov @vesselino.com [email protected] URL: www.vesselino.com 1 Starozagorska street, floor 3, office 24-23 6100 Kazanlak, BULGARIA tel./ +359 431 623 99, fax/ +359 431 81231 office mobile: +359 899 98 77 88 Oleum Occimum Basilicum Holly Basil 100.00 130.00 Oleum Origanum Heracleoticum Greek Oregano 100.00 155.00 ABSOLUTES Rosa damascena auct. -

Indiana Medical History Museum Guide to the Medicinal Plant Garden
Indiana Medical History Museum Guide to the Medicinal Plant Garden Garden created and maintained by Purdue Master Gardeners of Marion County IMHM Medicinal Plant Garden Plant List – Common Names Trees and Shrubs: Arborvitae, Thuja occidentalis Culver’s root, Veronicastrum virginicum Black haw, Viburnum prunifolium Day lily, Hemerocallis species Catalpa, Catalpa bignonioides Dill, Anethum graveolens Chaste tree, Vitex agnus-castus Elderberry, Sambucus nigra Dogwood, Cornus florida Elecampane, Inula helenium Elderberry, Sambucus nigra European meadowsweet, Queen of the meadow, Ginkgo, Ginkgo biloba Filipendula ulmaria Hawthorn, Crateagus oxycantha Evening primrose, Oenothera biennis Juniper, Juniperus communis False Solomon’s seal, Smilacina racemosa Redbud, Cercis canadensis Fennel, Foeniculum vulgare Sassafras, Sassafras albidum Feverfew, Tanacetum parthenium Spicebush, Lindera benzoin Flax, Linum usitatissimum Witch hazel, Hamamelis virginiana Foxglove, Digitalis species Garlic, Allium sativum Climbing Vines: Golden ragwort, Senecio aureus Grape, Vitis vinifera Goldenrod, Solidago species Hops, Humulus lupulus Horehound, Marrubium vulgare Passion flower, Maypop, Passiflora incarnata Hyssop, Hyssopus officinalis Wild yam, Dioscorea villosa Joe Pye weed, Eupatorium purpureum Ladybells, Adenophora species Herbaceous Plants: Lady’s mantle, Alchemilla vulgaris Alfalfa, Medicago sativa Lavender, Lavendula angustifolia Aloe vera, Aloe barbadensis Lemon balm, Melissa officinalis American skullcap, Scutellaria laterifolia Licorice, Glycyrrhiza -

Dictionary of Cultivated Plants and Their Regions of Diversity Second Edition Revised Of: A.C
Dictionary of cultivated plants and their regions of diversity Second edition revised of: A.C. Zeven and P.M. Zhukovsky, 1975, Dictionary of cultivated plants and their centres of diversity 'N -'\:K 1~ Li Dictionary of cultivated plants and their regions of diversity Excluding most ornamentals, forest trees and lower plants A.C. Zeven andJ.M.J, de Wet K pudoc Centre for Agricultural Publishing and Documentation Wageningen - 1982 ~T—^/-/- /+<>?- •/ CIP-GEGEVENS Zeven, A.C. Dictionary ofcultivate d plants andthei rregion so f diversity: excluding mostornamentals ,fores t treesan d lowerplant s/ A.C .Zeve n andJ.M.J ,d eWet .- Wageninge n : Pudoc. -11 1 Herz,uitg . van:Dictionar y of cultivatedplant s andthei r centreso fdiversit y /A.C .Zeve n andP.M . Zhukovsky, 1975.- Me t index,lit .opg . ISBN 90-220-0785-5 SISO63 2UD C63 3 Trefw.:plantenteelt . ISBN 90-220-0785-5 ©Centre forAgricultura l Publishing and Documentation, Wageningen,1982 . Nopar t of thisboo k mayb e reproduced andpublishe d in any form,b y print, photoprint,microfil m or any othermean swithou t written permission from thepublisher . Contents Preface 7 History of thewor k 8 Origins of agriculture anddomesticatio n ofplant s Cradles of agriculture and regions of diversity 21 1 Chinese-Japanese Region 32 2 Indochinese-IndonesianRegio n 48 3 Australian Region 65 4 Hindustani Region 70 5 Central AsianRegio n 81 6 NearEaster n Region 87 7 Mediterranean Region 103 8 African Region 121 9 European-Siberian Region 148 10 South American Region 164 11 CentralAmerica n andMexica n Region 185 12 NorthAmerica n Region 199 Specieswithou t an identified region 207 References 209 Indexo fbotanica l names 228 Preface The aimo f thiswor k ist ogiv e thereade r quick reference toth e regionso f diversity ofcultivate d plants.Fo r important crops,region so fdiversit y of related wild species areals opresented .Wil d species areofte nusefu l sources of genes to improve thevalu eo fcrops . -

Herbs 3352 N Service Dr
Growing Herbs 3352 N Service Dr. Red Wing, MN 55066 www.sargentsnursery.com Resource: U of M Extension P: 651-388-3847 E: [email protected] Herbs offer great versatility for the gardening enthusiast. While chiefly grown for seasoning foods, herbs have many other uses. Their oils and fragrances have long been valued in the manufacture of cosmetics, perfumes, dyes and potpourris. Their medicinal properties are currently the source of research worldwide. Herbal materials are also used in dried flower arrangements and related crafts. Many culinary herbs grown in Minnesota are members of two plant families. The mint family, Lamiaceae, includes basil, oregano, marjoram, catnip, all the mints, as well as rosemary, thyme, lavender, summer savory, and sage, are all grown for their aromatic leaves. Hardy perennials in this family can become invasive, especially mints. These plants tend to be bushy, and most have some tolerance of excessive heat and dry soil. The carrot family, Apiaceae includes dill, parsley, chervil, cilantro (also known as coriander), fennel, and lovage. They are all grown for foliage and some for seeds as well. These plants have a more upright, leggy habit, and require somewhat moister conditions, and deeper, looser soil. Common culinary herbs from other plant families include chives (Alliaceae), borage (Boraginaceae), tarragon (Asteraceae), and sorrel (Polygonaceae). Culture Soil Properties The majority of herbs demand a well drained soil with a pH range of 6.0-7.5 for successful growth. Outdoors, avoid planting in heavy clay soils as well as wet areas. Also, avoid soils that have a high nutrient content. These rich soils may actually prove detrimental to the herb’s quality by promoting rapid, lush growth that will contain only small amounts of the volatile oils that give herbs their characteristic aromas and flavors. -

Growing Herbs for the Home Gardener
Department of Horticultural Science Horticulture Information Leaflet 8110 2/98 GROWING HERBS FOR THE HOME GARDENER Erv Evans, Extension Associate, Horticultural Science Jeanine Davis, Extension Specialist, Horticultural Science An herb is any plant used whole or in part as • Plant herbs in average garden soil with an ingredient for health, flavor, or fragrance. organic matter added to improve texture Herbs can be used to make teas; perk up and drainage. cooked foods such as meats, vegetables, • Choose a site that receives at least 6 sauces, and soups; or to add flavor to hours of direct sun each day. vinegars, butters, dips, or mustards. Many herbs are grown for their fragrance and are • Avoid ground where water stands or used in potpourris, sachets, and nosegays; runs during heavy rains. or to scent bath water, candles, oils, or • Compensate for poor drainage with raised perfumes. More than 25% of our modern beds amended with compost. drugs contain plant extracts as active ingredients, and researchers continue to • Apply balanced fertilizers sparingly to isolate valuable new medicines from plants leafy, fast growing herbs. Heavy and confirm the benefits of those used in applications of fertilizer, especially those traditional folk medicine. containing large amounts of nitrogen, will decrease the concentration of essential Herbs as a group are relatively easy to grow. oils in the lush green growth. Begin your herb garden with the herbs you enjoy using the most. For example, choose Plan your herb garden by grouping herbs basil, oregano, and fennel for Italian cooking; according to light, irrigation, and soil lavender and lemon verbena for making requirements.