Structural Adaptations in Overwintering Leaves of Thermonastic and Nonthermonastic Rhododendron Species Xiang Wang Iowa State University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Department of Planning and Zoning
Department of Planning and Zoning Subject: Howard County Landscape Manual Updates: Recommended Street Tree List (Appendix B) and Recommended Plant List (Appendix C) - Effective July 1, 2010 To: DLD Review Staff Homebuilders Committee From: Kent Sheubrooks, Acting Chief Division of Land Development Date: July 1, 2010 Purpose: The purpose of this policy memorandum is to update the Recommended Plant Lists presently contained in the Landscape Manual. The plant lists were created for the first edition of the Manual in 1993 before information was available about invasive qualities of certain recommended plants contained in those lists (Norway Maple, Bradford Pear, etc.). Additionally, diseases and pests have made some other plants undesirable (Ash, Austrian Pine, etc.). The Howard County General Plan 2000 and subsequent environmental and community planning publications such as the Route 1 and Route 40 Manuals and the Green Neighborhood Design Guidelines have promoted the desirability of using native plants in landscape plantings. Therefore, this policy seeks to update the Recommended Plant Lists by identifying invasive plant species and disease or pest ridden plants for their removal and prohibition from further planting in Howard County and to add other available native plants which have desirable characteristics for street tree or general landscape use for inclusion on the Recommended Plant Lists. Please note that a comprehensive review of the street tree and landscape tree lists were conducted for the purpose of this update, however, only -

Central Appalachian Broadleaf Forest Coniferous Forest Meadow Province
Selecting Plants for Pollinators A Regional Guide for Farmers, Land Managers, and Gardeners In the Central Appalachian Broadleaf Forest Coniferous Forest Meadow Province Including the states of: Maryland, Pennsylvania, Virginia, West Virginia And parts of: Georgia, Kentucky, and North Carolina, NAPPC South Carolina, Tennessee Table of CONTENTS Why Support Pollinators? 4 Getting Started 5 Central Appalachian Broadleaf Forest 6 Meet the Pollinators 8 Plant Traits 10 Developing Plantings 12 Far ms 13 Public Lands 14 Home Landscapes 15 Bloom Periods 16 Plants That Attract Pollinators 18 Habitat Hints 20 This is one of several guides for Check list 22 different regions in the United States. We welcome your feedback to assist us in making the future Resources and Feedback 23 guides useful. Please contact us at [email protected] Cover: silver spotted skipper courtesy www.dangphoto.net 2 Selecting Plants for Pollinators Selecting Plants for Pollinators A Regional Guide for Farmers, Land Managers, and Gardeners In the Ecological Region of the Central Appalachian Broadleaf Forest Coniferous Forest Meadow Province Including the states of: Maryland, Pennsylvania, Virginia, West Virginia And parts of: Georgia, Kentucky, North Carolina, South Carolina, Tennessee a nappc and Pollinator Partnership™ Publication This guide was funded by the National Fish and Wildlife Foundation, the C.S. Fund, the Plant Conservation Alliance, the U.S. Forest Service, and the Bureau of Land Management with oversight by the Pollinator Partnership™ (www.pollinator.org), in support of the North American Pollinator Protection Campaign (NAPPC–www.nappc.org). Central Appalachian Broadleaf Forest – Coniferous Forest – Meadow Province 3 Why support pollinators? In theIr 1996 book, the Forgotten PollInators, Buchmann and Nabhan estimated that animal pollinators are needed for the reproduction “ Farming feeds of 90% of flowering plants and one third of human food crops. -

Rediscovering Rhododendron Dell, Part 2
Rediscovering Rhododendron Dell, Part 2 Kyle Port “They [hoodlums] deliberately twist off the metal labels from trees and shrubs, so that valuable information is sometimes lost forever and the yearly replacement bill is terrific. They break hundreds of unopened flower buds off the Rhododendrons in the early spring.” —Edgar Anderson, Arnold Arboretum arborist , June 4, 1932 ated C DI N I E S erwi H T SS O E UNL R HO T U A E H T Y B S E G A M I ALL Planted in close proximity to one another, Rhododendron ‘Old Port’ 990-56-B (a catawbiense hybrid with “vinous crimson” flowers, seen here) was incorrectly labeled as R. ‘Red Head’ 329-91-A (with “orient red” flowers). A description published by the Royal Horticultural Society was used to verify the only remaining plant as ‘Old Port’; a lack of indumentum on the undersides of the leaves distinguishes it from ‘Red Head’. he Arboretum’s plant records attest to curatorial review that has advanced our under- episodes of vandalism, arson, theft, and standing of the rhododendron collection and Tother willful shenanigans that have further fostered its use. occurred in the living collections over the years. In response to the identity crises in Rhodo- In 2010, a pile of plant record labels was found dendron Dell, a multi-year collection review in Rhododendron Dell. This intentional—and was conceived. Identity verification and field completely unsanctioned—removal of labels work (e.g., labeling, photographing) was timed from numerous specimens by an anonymous to coincide with peak flowering. -

Host Range of a Select Isolate of the Eri Coid Mycorrhizal Fungus
PROPAGATION & TISSUE CULTURE HORTSCIENCE 38(6):1163–1166. 2003. Berta and Bonfante-Fasolo, 1983; Bradley et al., 1981; Leake and Read, 1989). Other studies have attempted to evaluate the host Host Range of a Select Isolate of range of ericoid fungi, but have inoculated with unidentified ericoid fungal isolates, described, the Eri coid Mycorrhizal Fungus for example as, “dark, slow-growing cultures” (Pearson and Read, 1973b; Reed, 1987). Hymenoscyphus ericae To date there have been no studies that investigate the host range of a select isolate Nicole R. Gorman1 and Mark C. Starrett2 of the ericoid endophyte H. ericae. Therefore, Department of Plant and Soil Science, University of Vermont, Burlington, the objective of this study was to evaluate the VT 05405-0082 host range using select species within the Eri- caceae by inoculating with a specific iso late Additional index words. Ericaceae, Calluna, Enkianthus, Gaultheria, Kalmia, Leucothoe, of H. ericae. Oxydendrum, Pieris, Rhodo den dron, Vaccinium Materials and Methods Abstract. Studies were conducted to ex am ine the host range of a select isolate of the ericoid mycorrhizal fungus Hymenoscyphus ericae (Read) Korf and Kernan [American Type Seed of 15 ericaceous species was ob tained Culture Collection (ATCC) #32985]. Host status was tested for 15 ericaceous species, in- from commercial seed suppliers [Sheffield·s cluding: Calluna vulgaris (L.) Hull, Enkianthus campanulatus (Miq.) Nichols, Gaultheria Seed Co. (Lock, N.Y.) and F.W. Schumacher procumbens L., Kalmia latifolia L., Leucothoe fontanesiana Sleum., Oxydendrum arbo- Co. (Sandwich, Mass.)]. Seed was cleaned and reum (L.) DC., Pieris flo ri bun da (Pursh) Benth. -

Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- BIBLIOGRAPHY
Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- BIBLIOGRAPHY BIBLIOGRAPHY Ackerfield, J., and J. Wen. 2002. A morphometric analysis of Hedera L. (the ivy genus, Araliaceae) and its taxonomic implications. Adansonia 24: 197-212. Adams, P. 1961. Observations on the Sagittaria subulata complex. Rhodora 63: 247-265. Adams, R.M. II, and W.J. Dress. 1982. Nodding Lilium species of eastern North America (Liliaceae). Baileya 21: 165-188. Adams, R.P. 1986. Geographic variation in Juniperus silicicola and J. virginiana of the Southeastern United States: multivariant analyses of morphology and terpenoids. Taxon 35: 31-75. ------. 1995. Revisionary study of Caribbean species of Juniperus (Cupressaceae). Phytologia 78: 134-150. ------, and T. Demeke. 1993. Systematic relationships in Juniperus based on random amplified polymorphic DNAs (RAPDs). Taxon 42: 553-571. Adams, W.P. 1957. A revision of the genus Ascyrum (Hypericaceae). Rhodora 59: 73-95. ------. 1962. Studies in the Guttiferae. I. A synopsis of Hypericum section Myriandra. Contr. Gray Herbarium Harv. 182: 1-51. ------, and N.K.B. Robson. 1961. A re-evaluation of the generic status of Ascyrum and Crookea (Guttiferae). Rhodora 63: 10-16. Adams, W.P. 1973. Clusiaceae of the southeastern United States. J. Elisha Mitchell Sci. Soc. 89: 62-71. Adler, L. 1999. Polygonum perfoliatum (mile-a-minute weed). Chinquapin 7: 4. Aedo, C., J.J. Aldasoro, and C. Navarro. 1998. Taxonomic revision of Geranium sections Batrachioidea and Divaricata (Geraniaceae). Ann. Missouri Bot. Gard. 85: 594-630. Affolter, J.M. 1985. A monograph of the genus Lilaeopsis (Umbelliferae). Systematic Bot. Monographs 6. Ahles, H.E., and A.E. -

Introduction to the Southern Blue Ridge Ecoregional Conservation Plan
SOUTHERN BLUE RIDGE ECOREGIONAL CONSERVATION PLAN Summary and Implementation Document March 2000 THE NATURE CONSERVANCY and the SOUTHERN APPALACHIAN FOREST COALITION Southern Blue Ridge Ecoregional Conservation Plan Summary and Implementation Document Citation: The Nature Conservancy and Southern Appalachian Forest Coalition. 2000. Southern Blue Ridge Ecoregional Conservation Plan: Summary and Implementation Document. The Nature Conservancy: Durham, North Carolina. This document was produced in partnership by the following three conservation organizations: The Nature Conservancy is a nonprofit conservation organization with the mission to preserve plants, animals and natural communities that represent the diversity of life on Earth by protecting the lands and waters they need to survive. The Southern Appalachian Forest Coalition is a nonprofit organization that works to preserve, protect, and pass on the irreplaceable heritage of the region’s National Forests and mountain landscapes. The Association for Biodiversity Information is an organization dedicated to providing information for protecting the diversity of life on Earth. ABI is an independent nonprofit organization created in collaboration with the Network of Natural Heritage Programs and Conservation Data Centers and The Nature Conservancy, and is a leading source of reliable information on species and ecosystems for use in conservation and land use planning. Photocredits: Robert D. Sutter, The Nature Conservancy EXECUTIVE SUMMARY This first iteration of an ecoregional plan for the Southern Blue Ridge is a compendium of hypotheses on how to conserve species nearest extinction, rare and common natural communities and the rich and diverse biodiversity in the ecoregion. The plan identifies a portfolio of sites that is a vision for conservation action, enabling practitioners to set priorities among sites and develop site-specific and multi-site conservation strategies. -
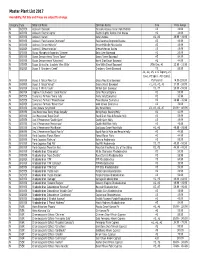
Master Plant List 2017.Xlsx
Master Plant List 2017 Availability, Pot Size and Prices are subject to change. Category Type Botanical Name Common Name Size Price Range N BREVER Azalea X 'Cascade' Cascade Azalea (Glenn Dale Hybrid) #3 49.99 N BREVER Azalea X 'Electric Lights' Electric Lights Double Pink Azalea #2 44.99 N BREVER Azalea X 'Karen' Karen Azalea #2, #3 39.99 - 49.99 N BREVER Azalea X 'Poukhanense Improved' Poukhanense Improved Azalea #3 49.99 N BREVER Azalea X 'Renee Michelle' Renee Michelle Pink Azalea #3 49.99 N BREVER Azalea X 'Stewartstonian' Stewartstonian Azalea #3 49.99 N BREVER Buxus Microphylla Japonica "Gregem' Baby Gem Boxwood #2 29.99 N BREVER Buxus Sempervirens 'Green Tower' Green Tower Boxwood #5 64.99 N BREVER Buxus Sempervirens 'Katerberg' North Star Dwarf Boxwood #2 44.99 N BREVER Buxus Sinica Var. Insularis 'Wee Willie' Wee Willie Dwarf Boxwood Little One, #1 13.99 - 21.99 N BREVER Buxus X 'Cranberry Creek' Cranberry Creek Boxwood #3 89.99 #1, #2, #5, #15 Topiary, #5 Cone, #5 Spiral, #10 Spiral, N BREVER Buxus X 'Green Mountain' Green Mountain Boxwood #5 Pyramid 14.99-299.99 N BREVER Buxus X 'Green Velvet' Green Velvet Boxwood #1, #2, #3, #5 17.99 - 59.99 N BREVER Buxus X 'Winter Gem' Winter Gem Boxwood #5, #7 59.99 - 99.99 N BREVER Daphne X Burkwoodii 'Carol Mackie' Carol Mackie Daphne #2 59.99 N BREVER Euonymus Fortunei 'Ivory Jade' Ivory Jade Euonymus #2 35.99 N BREVER Euonymus Fortunei 'Moonshadow' Moonshadow Euonymus #2 29.99 - 35.99 N BREVER Euonymus Fortunei 'Rosemrtwo' Gold Splash Euonymus #2 39.99 N BREVER Ilex Crenata 'Sky Pencil' -

Ozone Sensitive Plant Species on NPS and U.S. FWS Lands
Ozone Sensitive Plant Species on National Park Service and U.S. Fish and Wildlife Service Lands: Results of a June 24-25, 2003 Workshop Baltimore, Maryland National Park Service Air Resources Division U.S. Fish and Wildlife Service Air Quality Branch November 2003 Ozone Sensitive Plant Species on National Park Service and U.S. Fish and Wildlife Service Lands: Results of a June 24-25, 2003 Workshop Baltimore, Maryland Prepared by: Ellen Porter, Air Resources Division, National Park Service U.S. Department of the Interior National Park Service Air Resources Division, Denver, Colorado U.S. Fish and Wildlife Service Air Quality Branch, Denver, Colorado November 2003 NPS D1522 Natural Resource Report NPS/NRARD/NRR-2003/01 Acknowledgements: Drs. Art Chappelka, Howie Neufeld, Donald Davis, Robert Kohut and Pat Temple provided scientific expertise at the Baltimore Workshop. Dr. Gretchen Smith, Jim Renfro, Dr. David Peterson, Ed Jepsen, Dr. John Skelly, and Dr. William Manning provided additional scientific expertise and peer review. The author wishes to thank Tonnie Maniero, Tamara Blett, and Kristi Morris for helpful editing comments. This report is available at: www2.nature.nps.gov/ard/pubs/index.htm Cover photos by Dr. Donald Davis Contents Summary..................................................................................................1 Background ..............................................................................................1 Workshop Goals and Results .....................................................................3 -

The Red List of Rhododendrons
The Red List of Rhododendrons Douglas Gibbs, David Chamberlain and George Argent BOTANIC GARDENS CONSERVATION INTERNATIONAL (BGCI) is a membership organization linking botanic gardens in over 100 countries in a shared commitment to biodiversity conservation, sustainable use and environmental education. BGCI aims to mobilize botanic gardens and work with partners to secure plant diversity for the well-being of people and the planet. BGCI provides the Secretariat for the IUCN/SSC Global Tree Specialist Group. Published by Botanic Gardens Conservation FAUNA & FLORA INTERNATIONAL (FFI) , founded in 1903 and the International, Richmond, UK world’s oldest international conservation organization, acts to conserve © 2011 Botanic Gardens Conservation International threatened species and ecosystems worldwide, choosing solutions that are sustainable, are based on sound science and take account of ISBN: 978-1-905164-35-6 human needs. Reproduction of any part of the publication for educational, conservation and other non-profit purposes is authorized without prior permission from the copyright holder, provided that the source is fully acknowledged. Reproduction for resale or other commercial purposes is prohibited without prior written permission from the copyright holder. THE GLOBAL TREES CAMPAIGN is undertaken through a partnership between FFI and BGCI, working with a wide range of other The designation of geographical entities in this document and the presentation of the material do not organizations around the world, to save the world’s most threatened trees imply any expression on the part of the authors and the habitats in which they grow through the provision of information, or Botanic Gardens Conservation International delivery of conservation action and support for sustainable use. -

Ecological Zones in the Southern Appalachians: First Approximation
United States Department of Ecological Zones in the Southern Agriculture Forest Service Appalachians: First Approximation Steve A. Simon, Thomas K. Collins, Southern Gary L. Kauffman, W. Henry McNab, and Research Station Christopher J. Ulrey Research Paper SRS–41 The Authors Steven A. Simon, Ecologist, USDA Forest Service, National Forests in North Carolina, Asheville, NC 28802; Thomas K. Collins, Geologist, USDA Forest Service, George Washington and Jefferson National Forests, Roanoke, VA 24019; Gary L. Kauffman, Botanist, USDA Forest Service, National Forests in North Carolina, Asheville, NC 28802; W. Henry McNab, Research Forester, USDA Forest Service, Southern Research Station, Asheville, NC 28806; and Christopher J. Ulrey, Vegetation Specialist, U.S. Department of the Interior, National Park Service, Blue Ridge Parkway, Asheville, NC 28805. Cover Photos Ecological zones, regions of similar physical conditions and biological potential, are numerous and varied in the Southern Appalachian Mountains and are often typified by plant associations like the red spruce, Fraser fir, and northern hardwoods association found on the slopes of Mt. Mitchell (upper photo) and characteristic of high-elevation ecosystems in the region. Sites within ecological zones may be characterized by geologic formation, landform, aspect, and other physical variables that combine to form environments of varying temperature, moisture, and fertility, which are suitable to support characteristic species and forests, such as this Blue Ridge Parkway forest dominated by chestnut oak and pitch pine with an evergreen understory of mountain laurel (lower photo). DISCLAIMER The use of trade or firm names in this publication is for reader information and does not imply endorsement of any product or service by the U.S. -

Plant Taxonomy: What's in a Name?
Episode: Thewwwwwwwwwwwwwwwwwwwwww Naturalists EXPLORING NORTH CAROLINA Plant Taxonomy: What’s in a Name? STANDARD COURSE OF STUDY CORRELATIONS: Biology, Goal 4: The learner will develop an understanding of the unity and diversity of life. 4.01 Analyze the classification of organisms. Biology, Goal 5: The learner will develop an understanding of the ecological relationships among organisms. 5.03 Assess human population and its impact on local ecosystems and global environments. INTRODUCTION TO LESSON: Students will investigate the usefulness of taxonomy by deciphering the scientific names of North Carolina plants identi fied by explorer and naturalist André Michaux. They will complete a graphic organ MATERIALS izer using reference manuals, field guides and/or Internet resources to help them w Copies of assignment understand the significance of scientific names. sheet, one per pair of students BACKGROUND FOR TEACHER: Many early European explorers came to the New World w Viewing Guides, one looking for fairly common plants that were considered valuable natural resources. per student André Michaux was among the brave botanists who endured harrowing conditions w Reference books (see traveling through the unexplored backwoods. The French government sent Michaux Additional Resources) to North Carolina in 1785 to look for timber to supplement France’s depleted stocks. Michaux exceeded this mission, eventually sending 60,000 plant specimens back PREPARATION to France. In the Carolinas alone, he named and identified close to 300 plants that w Make copies of Viewing were new to science. Guide and Assignment Sheets. w If students will not have classroom Internet access, engage f Ask students to name some reasons that plants are useful. -

Genetic Diversity and Population Structure of Rhododendron Rex Subsp
plants Article Genetic Diversity and Population Structure of Rhododendron rex Subsp. rex Inferred from Microsatellite Markers and Chloroplast DNA Sequences 1,2,3,4, 1, 1,2,3 1,2,3, Xue Zhang y, Yuan-Huan Liu y, Yue-Hua Wang and Shi-Kang Shen * 1 School of Life Sciences, Yunnan University, Kunming 650091, China; [email protected] (X.Z.); [email protected] (Y.-H.L.); [email protected] (Y.-H.W.) 2 School of Ecology and Environmental Sciences & Yunnan Key Laboratory for Plateau Mountain Ecology and Restoration of Degraded Environments, Yunnan University, Kunming 650091, China 3 Yunnan Key Laboratory of Plant Reproductive Adaptation and Evolutionary Ecology, Yunnan University, Kunming 650091, China 4 Breeding Base for State Key Laboratory of Land Degradation and Ecological Restoration in Northwest China, Key Laboratory for Restoration and Reconstruction of Degraded Ecosystem in Northwest China of Ministry of Education, Ningxia University, Yinchuan 750021, China * Correspondence: [email protected]; Tel.: +86-871-65031412 These authors contribute equals to this work. y Received: 7 February 2020; Accepted: 5 March 2020; Published: 7 March 2020 Abstract: Genetic diversity is vital to the sustainable utilization and conservation of plant species. Rhododendron rex subsp. rex Lévl. is an endangered species endemic to the southwest of China. Although the natural populations of this species are facing continuous decline due to the high frequency of anthropogenic disturbance, the genetic information of R. rex subsp. rex is not yet elucidated. In the present study, 10 pairs of microsatellite markers (nSSRs) and three pairs of chloroplast DNA (cpDNAs) were used in the elucidation of the genetic diversity, population structure, and demographic history of 11 R.