Long-Term Demography, Microclimatic Refugia, and Responses to Climate Change for a High-Elevation Southern Appalachian Endemic Plant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Department of Planning and Zoning
Department of Planning and Zoning Subject: Howard County Landscape Manual Updates: Recommended Street Tree List (Appendix B) and Recommended Plant List (Appendix C) - Effective July 1, 2010 To: DLD Review Staff Homebuilders Committee From: Kent Sheubrooks, Acting Chief Division of Land Development Date: July 1, 2010 Purpose: The purpose of this policy memorandum is to update the Recommended Plant Lists presently contained in the Landscape Manual. The plant lists were created for the first edition of the Manual in 1993 before information was available about invasive qualities of certain recommended plants contained in those lists (Norway Maple, Bradford Pear, etc.). Additionally, diseases and pests have made some other plants undesirable (Ash, Austrian Pine, etc.). The Howard County General Plan 2000 and subsequent environmental and community planning publications such as the Route 1 and Route 40 Manuals and the Green Neighborhood Design Guidelines have promoted the desirability of using native plants in landscape plantings. Therefore, this policy seeks to update the Recommended Plant Lists by identifying invasive plant species and disease or pest ridden plants for their removal and prohibition from further planting in Howard County and to add other available native plants which have desirable characteristics for street tree or general landscape use for inclusion on the Recommended Plant Lists. Please note that a comprehensive review of the street tree and landscape tree lists were conducted for the purpose of this update, however, only -

Central Appalachian Broadleaf Forest Coniferous Forest Meadow Province
Selecting Plants for Pollinators A Regional Guide for Farmers, Land Managers, and Gardeners In the Central Appalachian Broadleaf Forest Coniferous Forest Meadow Province Including the states of: Maryland, Pennsylvania, Virginia, West Virginia And parts of: Georgia, Kentucky, and North Carolina, NAPPC South Carolina, Tennessee Table of CONTENTS Why Support Pollinators? 4 Getting Started 5 Central Appalachian Broadleaf Forest 6 Meet the Pollinators 8 Plant Traits 10 Developing Plantings 12 Far ms 13 Public Lands 14 Home Landscapes 15 Bloom Periods 16 Plants That Attract Pollinators 18 Habitat Hints 20 This is one of several guides for Check list 22 different regions in the United States. We welcome your feedback to assist us in making the future Resources and Feedback 23 guides useful. Please contact us at [email protected] Cover: silver spotted skipper courtesy www.dangphoto.net 2 Selecting Plants for Pollinators Selecting Plants for Pollinators A Regional Guide for Farmers, Land Managers, and Gardeners In the Ecological Region of the Central Appalachian Broadleaf Forest Coniferous Forest Meadow Province Including the states of: Maryland, Pennsylvania, Virginia, West Virginia And parts of: Georgia, Kentucky, North Carolina, South Carolina, Tennessee a nappc and Pollinator Partnership™ Publication This guide was funded by the National Fish and Wildlife Foundation, the C.S. Fund, the Plant Conservation Alliance, the U.S. Forest Service, and the Bureau of Land Management with oversight by the Pollinator Partnership™ (www.pollinator.org), in support of the North American Pollinator Protection Campaign (NAPPC–www.nappc.org). Central Appalachian Broadleaf Forest – Coniferous Forest – Meadow Province 3 Why support pollinators? In theIr 1996 book, the Forgotten PollInators, Buchmann and Nabhan estimated that animal pollinators are needed for the reproduction “ Farming feeds of 90% of flowering plants and one third of human food crops. -

Natural Heritage Program List of Rare Plant Species of North Carolina 2016
Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Revised February 24, 2017 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org C ur Alleghany rit Ashe Northampton Gates C uc Surry am k Stokes P d Rockingham Caswell Person Vance Warren a e P s n Hertford e qu Chowan r Granville q ot ui a Mountains Watauga Halifax m nk an Wilkes Yadkin s Mitchell Avery Forsyth Orange Guilford Franklin Bertie Alamance Durham Nash Yancey Alexander Madison Caldwell Davie Edgecombe Washington Tyrrell Iredell Martin Dare Burke Davidson Wake McDowell Randolph Chatham Wilson Buncombe Catawba Rowan Beaufort Haywood Pitt Swain Hyde Lee Lincoln Greene Rutherford Johnston Graham Henderson Jackson Cabarrus Montgomery Harnett Cleveland Wayne Polk Gaston Stanly Cherokee Macon Transylvania Lenoir Mecklenburg Moore Clay Pamlico Hoke Union d Cumberland Jones Anson on Sampson hm Duplin ic Craven Piedmont R nd tla Onslow Carteret co S Robeson Bladen Pender Sandhills Columbus New Hanover Tidewater Coastal Plain Brunswick THE COUNTIES AND PHYSIOGRAPHIC PROVINCES OF NORTH CAROLINA Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org This list is dynamic and is revised frequently as new data become available. New species are added to the list, and others are dropped from the list as appropriate. -

Rediscovering Rhododendron Dell, Part 2
Rediscovering Rhododendron Dell, Part 2 Kyle Port “They [hoodlums] deliberately twist off the metal labels from trees and shrubs, so that valuable information is sometimes lost forever and the yearly replacement bill is terrific. They break hundreds of unopened flower buds off the Rhododendrons in the early spring.” —Edgar Anderson, Arnold Arboretum arborist , June 4, 1932 ated C DI N I E S erwi H T SS O E UNL R HO T U A E H T Y B S E G A M I ALL Planted in close proximity to one another, Rhododendron ‘Old Port’ 990-56-B (a catawbiense hybrid with “vinous crimson” flowers, seen here) was incorrectly labeled as R. ‘Red Head’ 329-91-A (with “orient red” flowers). A description published by the Royal Horticultural Society was used to verify the only remaining plant as ‘Old Port’; a lack of indumentum on the undersides of the leaves distinguishes it from ‘Red Head’. he Arboretum’s plant records attest to curatorial review that has advanced our under- episodes of vandalism, arson, theft, and standing of the rhododendron collection and Tother willful shenanigans that have further fostered its use. occurred in the living collections over the years. In response to the identity crises in Rhodo- In 2010, a pile of plant record labels was found dendron Dell, a multi-year collection review in Rhododendron Dell. This intentional—and was conceived. Identity verification and field completely unsanctioned—removal of labels work (e.g., labeling, photographing) was timed from numerous specimens by an anonymous to coincide with peak flowering. -

Structural Adaptations in Overwintering Leaves of Thermonastic and Nonthermonastic Rhododendron Species Xiang Wang Iowa State University
Genetics, Development and Cell Biology Genetics, Development and Cell Biology Publications 11-2008 Structural Adaptations in Overwintering Leaves of Thermonastic and Nonthermonastic Rhododendron Species Xiang Wang Iowa State University Rajeev Arora Iowa State University, [email protected] Harry T. Horner Iowa State University, [email protected] Stephen L. Krebs David G. Leach Station of the Holden Arboretum Follow this and additional works at: http://lib.dr.iastate.edu/gdcb_las_pubs Part of the Horticulture Commons, Other Plant Sciences Commons, Plant Biology Commons, and the Plant Breeding and Genetics Commons The ompc lete bibliographic information for this item can be found at http://lib.dr.iastate.edu/ gdcb_las_pubs/47. For information on how to cite this item, please visit http://lib.dr.iastate.edu/ howtocite.html. This Article is brought to you for free and open access by the Genetics, Development and Cell Biology at Iowa State University Digital Repository. It has been accepted for inclusion in Genetics, Development and Cell Biology Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Structural Adaptations in Overwintering Leaves of Thermonastic and Nonthermonastic Rhododendron Species Abstract Evergreen rhododendrons (Rhododendron L.) are important woody landscape plants in many temperate zones. During winters, leaves of these plants frequently are exposed to a combination of cold temperatures, high radiation, and reduced photosynthetic activity, conditions that render them vulnerable to photooxidative damage. In addition, these plants are shallow-rooted and thus susceptible to leaf desiccation when soils are frozen. In this study, the potential adaptive significance of leaf morphology and anatomy in two contrasting Rhododendron species was investigated. -

Host Range of a Select Isolate of the Eri Coid Mycorrhizal Fungus
PROPAGATION & TISSUE CULTURE HORTSCIENCE 38(6):1163–1166. 2003. Berta and Bonfante-Fasolo, 1983; Bradley et al., 1981; Leake and Read, 1989). Other studies have attempted to evaluate the host Host Range of a Select Isolate of range of ericoid fungi, but have inoculated with unidentified ericoid fungal isolates, described, the Eri coid Mycorrhizal Fungus for example as, “dark, slow-growing cultures” (Pearson and Read, 1973b; Reed, 1987). Hymenoscyphus ericae To date there have been no studies that investigate the host range of a select isolate Nicole R. Gorman1 and Mark C. Starrett2 of the ericoid endophyte H. ericae. Therefore, Department of Plant and Soil Science, University of Vermont, Burlington, the objective of this study was to evaluate the VT 05405-0082 host range using select species within the Eri- caceae by inoculating with a specific iso late Additional index words. Ericaceae, Calluna, Enkianthus, Gaultheria, Kalmia, Leucothoe, of H. ericae. Oxydendrum, Pieris, Rhodo den dron, Vaccinium Materials and Methods Abstract. Studies were conducted to ex am ine the host range of a select isolate of the ericoid mycorrhizal fungus Hymenoscyphus ericae (Read) Korf and Kernan [American Type Seed of 15 ericaceous species was ob tained Culture Collection (ATCC) #32985]. Host status was tested for 15 ericaceous species, in- from commercial seed suppliers [Sheffield·s cluding: Calluna vulgaris (L.) Hull, Enkianthus campanulatus (Miq.) Nichols, Gaultheria Seed Co. (Lock, N.Y.) and F.W. Schumacher procumbens L., Kalmia latifolia L., Leucothoe fontanesiana Sleum., Oxydendrum arbo- Co. (Sandwich, Mass.)]. Seed was cleaned and reum (L.) DC., Pieris flo ri bun da (Pursh) Benth. -

THE GENETIC DIVERSITY and POPULATION STRUCTURE of GEUM RADIATUM: EFFECTS of NATURAL HISTORY and CONSERVATION EFFORTS a Thesis B
THE GENETIC DIVERSITY AND POPULATION STRUCTURE OF GEUM RADIATUM: EFFECTS OF NATURAL HISTORY AND CONSERVATION EFFORTS A Thesis by NIKOLAI M. HAY Submitted to the Graduate School at Appalachian State University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE December 2017 Department of Biology ! THE GENETIC DIVERSITY AND POPULATION STRUCTURE OF GEUM RADIATUM: EFFECTS OF NATURAL HISTORY AND CONSERVATION EFFORTS A Thesis by NIKOLAI M. HAY December 2017 APPROVED BY: Matt C. Estep Chairperson, Thesis Committee Zack E. Murrell Member, Thesis Committee Ray Williams Member, Thesis Committee Zack E Murrell Chairperson, Department of Biology Max C. Poole, Ph.D. Dean, Cratis D. Williams School of Graduate Studies ! Copyright by Nikolai M. Hay 2017 All Rights Reserved ! Abstract THE GENETIC DIVERSITY AND POPULATION STRUCTURE OF GEUM RADIATUM: EFFECTS OF NATURAL HISTORY AND CONSERVATION EFFORTS Nikolai M. Hay B.S., Appalachian State University M.S., Appalachian State University Chairperson: Matt C. Estep Geum radiatum is a federally endangered high-elevation rock outcrop endemic herb that is widely recognized as a hexaploid and a relic species. Little is currently known about G. radiatum genetic diversity, population interactions, or the effect of augmentations. This study sampled every known population of G. radiatum and used microsatellite markers to observe the alleles present at 8 loci. F-statistics, STRUCTURE, GENODIVE, and the R package polysat were used to measure diversity and genetic structure. The analysis demonstrates that there is interconnectedness and structure of populations and was able to locate augmented and punitive hybrids individuals within an augmented population. Geum radiautm has diversity among and between populations and suggests current gene flow in the northern populations. -

Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- BIBLIOGRAPHY
Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- BIBLIOGRAPHY BIBLIOGRAPHY Ackerfield, J., and J. Wen. 2002. A morphometric analysis of Hedera L. (the ivy genus, Araliaceae) and its taxonomic implications. Adansonia 24: 197-212. Adams, P. 1961. Observations on the Sagittaria subulata complex. Rhodora 63: 247-265. Adams, R.M. II, and W.J. Dress. 1982. Nodding Lilium species of eastern North America (Liliaceae). Baileya 21: 165-188. Adams, R.P. 1986. Geographic variation in Juniperus silicicola and J. virginiana of the Southeastern United States: multivariant analyses of morphology and terpenoids. Taxon 35: 31-75. ------. 1995. Revisionary study of Caribbean species of Juniperus (Cupressaceae). Phytologia 78: 134-150. ------, and T. Demeke. 1993. Systematic relationships in Juniperus based on random amplified polymorphic DNAs (RAPDs). Taxon 42: 553-571. Adams, W.P. 1957. A revision of the genus Ascyrum (Hypericaceae). Rhodora 59: 73-95. ------. 1962. Studies in the Guttiferae. I. A synopsis of Hypericum section Myriandra. Contr. Gray Herbarium Harv. 182: 1-51. ------, and N.K.B. Robson. 1961. A re-evaluation of the generic status of Ascyrum and Crookea (Guttiferae). Rhodora 63: 10-16. Adams, W.P. 1973. Clusiaceae of the southeastern United States. J. Elisha Mitchell Sci. Soc. 89: 62-71. Adler, L. 1999. Polygonum perfoliatum (mile-a-minute weed). Chinquapin 7: 4. Aedo, C., J.J. Aldasoro, and C. Navarro. 1998. Taxonomic revision of Geranium sections Batrachioidea and Divaricata (Geraniaceae). Ann. Missouri Bot. Gard. 85: 594-630. Affolter, J.M. 1985. A monograph of the genus Lilaeopsis (Umbelliferae). Systematic Bot. Monographs 6. Ahles, H.E., and A.E. -

Introduction to the Southern Blue Ridge Ecoregional Conservation Plan
SOUTHERN BLUE RIDGE ECOREGIONAL CONSERVATION PLAN Summary and Implementation Document March 2000 THE NATURE CONSERVANCY and the SOUTHERN APPALACHIAN FOREST COALITION Southern Blue Ridge Ecoregional Conservation Plan Summary and Implementation Document Citation: The Nature Conservancy and Southern Appalachian Forest Coalition. 2000. Southern Blue Ridge Ecoregional Conservation Plan: Summary and Implementation Document. The Nature Conservancy: Durham, North Carolina. This document was produced in partnership by the following three conservation organizations: The Nature Conservancy is a nonprofit conservation organization with the mission to preserve plants, animals and natural communities that represent the diversity of life on Earth by protecting the lands and waters they need to survive. The Southern Appalachian Forest Coalition is a nonprofit organization that works to preserve, protect, and pass on the irreplaceable heritage of the region’s National Forests and mountain landscapes. The Association for Biodiversity Information is an organization dedicated to providing information for protecting the diversity of life on Earth. ABI is an independent nonprofit organization created in collaboration with the Network of Natural Heritage Programs and Conservation Data Centers and The Nature Conservancy, and is a leading source of reliable information on species and ecosystems for use in conservation and land use planning. Photocredits: Robert D. Sutter, The Nature Conservancy EXECUTIVE SUMMARY This first iteration of an ecoregional plan for the Southern Blue Ridge is a compendium of hypotheses on how to conserve species nearest extinction, rare and common natural communities and the rich and diverse biodiversity in the ecoregion. The plan identifies a portfolio of sites that is a vision for conservation action, enabling practitioners to set priorities among sites and develop site-specific and multi-site conservation strategies. -
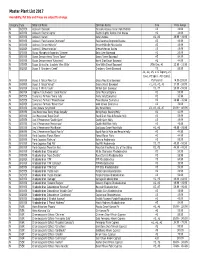
Master Plant List 2017.Xlsx
Master Plant List 2017 Availability, Pot Size and Prices are subject to change. Category Type Botanical Name Common Name Size Price Range N BREVER Azalea X 'Cascade' Cascade Azalea (Glenn Dale Hybrid) #3 49.99 N BREVER Azalea X 'Electric Lights' Electric Lights Double Pink Azalea #2 44.99 N BREVER Azalea X 'Karen' Karen Azalea #2, #3 39.99 - 49.99 N BREVER Azalea X 'Poukhanense Improved' Poukhanense Improved Azalea #3 49.99 N BREVER Azalea X 'Renee Michelle' Renee Michelle Pink Azalea #3 49.99 N BREVER Azalea X 'Stewartstonian' Stewartstonian Azalea #3 49.99 N BREVER Buxus Microphylla Japonica "Gregem' Baby Gem Boxwood #2 29.99 N BREVER Buxus Sempervirens 'Green Tower' Green Tower Boxwood #5 64.99 N BREVER Buxus Sempervirens 'Katerberg' North Star Dwarf Boxwood #2 44.99 N BREVER Buxus Sinica Var. Insularis 'Wee Willie' Wee Willie Dwarf Boxwood Little One, #1 13.99 - 21.99 N BREVER Buxus X 'Cranberry Creek' Cranberry Creek Boxwood #3 89.99 #1, #2, #5, #15 Topiary, #5 Cone, #5 Spiral, #10 Spiral, N BREVER Buxus X 'Green Mountain' Green Mountain Boxwood #5 Pyramid 14.99-299.99 N BREVER Buxus X 'Green Velvet' Green Velvet Boxwood #1, #2, #3, #5 17.99 - 59.99 N BREVER Buxus X 'Winter Gem' Winter Gem Boxwood #5, #7 59.99 - 99.99 N BREVER Daphne X Burkwoodii 'Carol Mackie' Carol Mackie Daphne #2 59.99 N BREVER Euonymus Fortunei 'Ivory Jade' Ivory Jade Euonymus #2 35.99 N BREVER Euonymus Fortunei 'Moonshadow' Moonshadow Euonymus #2 29.99 - 35.99 N BREVER Euonymus Fortunei 'Rosemrtwo' Gold Splash Euonymus #2 39.99 N BREVER Ilex Crenata 'Sky Pencil' -

Ozone Sensitive Plant Species on NPS and U.S. FWS Lands
Ozone Sensitive Plant Species on National Park Service and U.S. Fish and Wildlife Service Lands: Results of a June 24-25, 2003 Workshop Baltimore, Maryland National Park Service Air Resources Division U.S. Fish and Wildlife Service Air Quality Branch November 2003 Ozone Sensitive Plant Species on National Park Service and U.S. Fish and Wildlife Service Lands: Results of a June 24-25, 2003 Workshop Baltimore, Maryland Prepared by: Ellen Porter, Air Resources Division, National Park Service U.S. Department of the Interior National Park Service Air Resources Division, Denver, Colorado U.S. Fish and Wildlife Service Air Quality Branch, Denver, Colorado November 2003 NPS D1522 Natural Resource Report NPS/NRARD/NRR-2003/01 Acknowledgements: Drs. Art Chappelka, Howie Neufeld, Donald Davis, Robert Kohut and Pat Temple provided scientific expertise at the Baltimore Workshop. Dr. Gretchen Smith, Jim Renfro, Dr. David Peterson, Ed Jepsen, Dr. John Skelly, and Dr. William Manning provided additional scientific expertise and peer review. The author wishes to thank Tonnie Maniero, Tamara Blett, and Kristi Morris for helpful editing comments. This report is available at: www2.nature.nps.gov/ard/pubs/index.htm Cover photos by Dr. Donald Davis Contents Summary..................................................................................................1 Background ..............................................................................................1 Workshop Goals and Results .....................................................................3 -

Federally-Listed Wildlife Species
Assessment for the Nantahala and Pisgah NFs March 2014 Federally-Listed Wildlife Species Ten federally-endangered (E) or threatened (T) wildlife species are known to occur on or immediately adjacent to the Nantahala and Pisgah National Forests (hereafter, the Nantahala and Pisgah NFs). These include four small mammals, two terrestrial invertebrates, three freshwater mussels, and one fish (Table 1). Additionally, two endangered species historically occurred on or adjacent to the Forest, but are considered extirpated, or absent, from North Carolina and are no longer tracked by the North Carolina Natural Heritage Program (Table 1). Table 1. Federally-listed wildlife species known to occur or historically occurring on or immediately adjacent to the Nantahala and Pisgah National Forests. Common Name Scientific Name Federal Status Small Mammals Carolina northern flying Glaucomys sabrinus coloratus Endangered squirrel Gray myotis Myotis grisescens Endangered Virginia big-eared bat Corynorhinus townsendii Endangered virginianus Northern long-eared bat Myotis septentrionalis Endangered* Indiana bat Myotis sodalis Endangered Terrestrial Invertebrates Spruce-fir moss spider Microhexura montivaga Endangered noonday globe Patera clarki Nantahala Threatened Freshwater Mussels Appalachian elktoe Alasmidonta raveneliana Endangered Little-wing pearlymussel Pegius fabula Endangered Cumberland bean Villosa trabilis Endangered Spotfin chub Erimonax monachus Threatened Species Considered Extirpated From North Carolina American burying beetle Nicrophorous americanus Endangered Eastern cougar Puma concolor cougar Endangered *Pending final listing following the 12-month finding published in the Federal Register, October 2, 2013. Additionally, the United States Fish and Wildlife Service (USFWS) is addressing petitions to federally list two aquatic species known to occur on or immediately adjacent to Nantahala and Pisgah NFs: eastern hellbender (Cryptobranchus alleganiensis alleganiensis), a large aquatic salamander, and sicklefin redhorse (Moxostoma species 2), a fish.