Retinal Cotton-Wool Spots: an Early Finding in Diabetic Retinopathy?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Care Label Recommendations
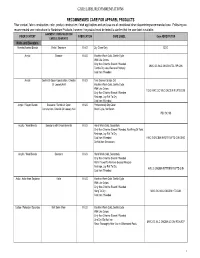
CARE LABEL RECOMMENDATIONS RECOMMENDED CARE FOR APPAREL PRODUCTS Fiber content, fabric construction, color, product construction, finish applications and end use are all considered when determining recommended care. Following are recommended care instructions for Nordstrom Products, however; the product must be tested to confirm that the care label is suitable. GARMENT/ CONSTRUCTION/ FIBER CONTENT FABRICATION CARE LABEL Care ABREVIATION EMBELLISHMENTS Knits and Sweaters Acetate/Acetate Blends Knits / Sweaters K & S Dry Clean Only DCO Acrylic Sweater K & S Machine Wash Cold, Gentle Cycle With Like Colors Only Non-Chlorine Bleach If Needed MWC GC WLC ONCBIN TDL RP CIIN Tumble Dry Low, Remove Promptly Cool Iron If Needed Acrylic Gentle Or Open Construction, Chenille K & S Turn Garment Inside Out Or Loosely Knit Machine Wash Cold, Gentle Cycle With Like Colors TGIO MWC GC WLC ONCBIN R LFTD CIIN Only Non-Chlorine Bleach If Needed Reshape, Lay Flat To Dry Cool Iron If Needed Acrylic / Rayon Blends Sweaters / Gentle Or Open K & S Professionally Dry Clean Construction, Chenille Or Loosely Knit Short Cycle, No Steam PDC SC NS Acrylic / Wool Blends Sweaters with Embelishments K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed, No Wring Or Twist Reshape, Lay Flat To Dry Cool Iron If Needed HWC S ONCBIN NWOT R LFTD CIIN DNID Do Not Iron Decoration Acrylic / Wool Blends Sweaters K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed Roll In Towel To Remove Excess Moisture Reshape, Lay Flat To Dry HWC S ONCBIN RITTREM -

Textile Printing
TECHNICAL BULLETIN 6399 Weston Parkway, Cary, North Carolina, 27513 • Telephone (919) 678-2220 ISP 1004 TEXTILE PRINTING This report is sponsored by the Importer Support Program and written to address the technical needs of product sourcers. © 2003 Cotton Incorporated. All rights reserved; America’s Cotton Producers and Importers. INTRODUCTION The desire of adding color and design to textile materials is almost as old as mankind. Early civilizations used color and design to distinguish themselves and to set themselves apart from others. Textile printing is the most important and versatile of the techniques used to add design, color, and specialty to textile fabrics. It can be thought of as the coloring technique that combines art, engineering, and dyeing technology to produce textile product images that had previously only existed in the imagination of the textile designer. Textile printing can realistically be considered localized dyeing. In ancient times, man sought these designs and images mainly for clothing or apparel, but in today’s marketplace, textile printing is important for upholstery, domestics (sheets, towels, draperies), floor coverings, and numerous other uses. The exact origin of textile printing is difficult to determine. However, a number of early civilizations developed various techniques for imparting color and design to textile garments. Batik is a modern art form for developing unique dyed patterns on textile fabrics very similar to textile printing. Batik is characterized by unique patterns and color combinations as well as the appearance of fracture lines due to the cracking of the wax during the dyeing process. Batik is derived from the Japanese term, “Ambatik,” which means “dabbing,” “writing,” or “drawing.” In Egypt, records from 23-79 AD describe a hot wax technique similar to batik. -
Multimodal Imaging of Cotton Wool Spots in Branch Retinal Vein Occlusion
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Bern Open Repository and Information System (BORIS) Original Paper Ophthalmic Res 2015;54:48–56 Received: April 7, 2015 DOI: 10.1159/000430843 Accepted: April 20, 2015 Published online: June 12, 2015 Multimodal Imaging of Cotton Wool Spots in Branch Retinal Vein Occlusion a, b, e a, b, f a, b Marion R. Munk Gerlinde Matt Magdalena Baratsits a, b c a, d a, b Roman Dunavoelgyi Wolfgang Huf Alessio Montuoro Wolf Buehl a, b a, b Ursula Schmidt-Erfurth Stefan Sacu on behalf of the Macula Study Group a b c Department of Ophthalmology, Medical University of Vienna, Center for Medical Physics and Biomedical d e Engineering and Vienna Reading Center, Medical University of Vienna, Vienna , Austria; Department of f Ophthalmology, Inselspital, University Hospital Bern, Bern , and Department of Ophthalmology, University Hospital Zurich, Zurich , Switzerland Key Words er than on CF (0.26 ± 0.17 mm 2 vs. 0.13 ± 0.1 mm 2 , p < 0.0001). Ischemia · Cotton wool spots · Cystoid bodies · Argon The CWS area on SD-OCT and surrounding pathology such laser treatment · Retinal vein occlusion · Branch retinal as intraretinal cysts, avascular zones and intraretinal hemor- vein occlusion · Hypoperfusion · Optical coherence rhage were predictive for how long CWS remained visible tomography · Fluorescein angiography · Axoplasmic (r 2 = 0.497, p < 0.002). Conclusions: The lifetime and presen- debris · Multimodal imaging · Acute macular tation of CWS in BRVO seem comparable to other diseases. neuroretinopathy SD-OCT shows a higher sensitivity for detecting CWS com- pared to CF. -

Material/Source Features Pros Cons Polyester/Cotton Blend 80% Polyester / 20% Cotton, 65/35, and 40/60 Are Common Blends. • La
Material/Source Features Pros Cons Polyester/Cotton Blend Liquid Resistance Appropriate for use in Polyester blends burn Splash resistant. clinical settings and readily when ignited, 80% Polyester / 20% No specific chemical resistance. research laboratories and are not appropriate Cotton, 65/35, and 40/60 Anecdotal evidence suggests polyester blends provide better protection against where biological for use with flammable are common blends. corrosive material than does cotton. material is liquids, pyrophoric Flame Resistance manipulated. materials, or near open No flame. Lab Supply Polyester blends burn more readily than 100% cotton or flame-resistant Companies materials. Chemistry Comfort Stockroom Lightweight and breathable. Biology More cotton in the blend results in better breathability. Stockroom 100% Cotton Liquid Resistance Appropriate for use in Cotton lab coats should Not splash resistant. clinical settings and be supplemented with a Lab Supply No specific chemical resistance. research laboratories chemical splash apron Companies Anecdotal evidence suggests cotton lab coats provide better protection from where there is light when corrosive material Chemistry solvent contamination than corrosive contamination. flammable liquid or is handled. Stockroom Flame Resistance open flame use. Biology No Stockroom Burns less readily than polyester blends. Comfort Lightweight and breathable. 100% Cotton treated with Liquid Resistance Appropriate for use in More costly than a flame retardant. Not splash resistant. research laboratories traditional 100% cotton No specific chemical resistance. where substantial fire lab coat. Lab Supply Anecdotal evidence suggests cotton lab coats provide better protection from risk exists from Companies solvent contamination than corrosive contamination. flammable material Manufacturers of Flame Resistance handling or open flame flame-resistant Yes use. -

27 Cardiovascular Disorders
27 Cardiovascular Disorders ◆ Systemic Hypertension Effects of Systemic Hypertension on the Eye and Vision ◆ Hypertensive retinopathy ◆ Hypertensive choroidopathy ◆ Hypertensive optic neuropathy ◆ Central and branch retinal vein occlusion ◆ Retinal macroaneurysm ◆ Vitreous hemorrhage ◆ Subconjunctival hemorrhage ◆ Secondary effects ° Carotid disease—retinal arterial emboli ° Cerebrovascular accident—visual field loss ° Ischemic optic neuropathy ° Oculomotor cranial nerve palsies ° Cataract, glaucoma, age-related macular degeneration Hypertensive Retinopathy Hypertensive retinopathy is usually asymptomatic. In severe hypertension, how- ever, painless loss of vision may occur due to the development of vitreous or reti- nal hemorrhages, retinal edema, retinal vascular occlusion, serous retinal detach- ment, choroidal ischemia, or optic neuropathy. Fundus Features ◆ Focal or diffuse narrowing of retinal arterioles ◆ Increased vascular tortuosity ( Fig. 27.1 ) ◆ Arteriovenous nicking (nipping) ( Fig. 27.2 ) ◆ Retinal hemorrhages ( Fig. 27.3 ) ° Flame-shaped hemorrhage ° Dot and blot hemorrhage ° Preretinal hemorrhage ◆ Microaneurysms ◆ Hard exudates, macular star ◆ Cotton-wool spots (F igs. 27.4 and 27.5 ) ◆ Abnormality of vascular reflex ° Copper-wire reflex ( Fig. 27.6 ) ° Silver-wire reflex ( Fig. 27.7 ) ◆ Intraretinal microvascular abnormalities ◆ Retinal edema ( Fig. 27.8 ) ◆ Serous retinal detachment—occurs secondary to hypertensive choroidopathy in patients with very severe hypertension or eclampsia ( Fig. 27.9 ) ◆ Elschnig spots—small to medium-size hyper- and hypopigmented patches rep- resenting chorioretinal scarring due to prior choroidal infarction ( Figs. 27.10 and 27.11 ) 111 14585C27.indd 111 7/8/09 3:44:46 PM 112 III The Retina in Systemic Disease ◆ Siegrist streaks—linear configurations of hyperpigmentation that have a patho- genesis similar to that of Elschnig spots. ◆ Optic disc edema ( Fig. 27.12 ) ◆ Optic atrophy ◆ Vitreous hemorrhage Fig. -

Hypertension and the Eye
rnib.org.uk/gp [email protected] Hypertension and the eye This factsheet was produced under a collaboration between the UK Vision Strategy, RNIB, and the Royal College of General Practitioners. Key learning points • Hypertensive retinopathy is a clinical diagnosis made when characteristic fundus findings are seen in a patient with or who has had systemic arterial hypertension. • Mild hypertensive retinal features are seen commonly and are of limited relevance, advanced changes represent important signs of accelerated hypertension. • The main complications from hypertension are retinal artery and retinal vein occlusions, and these cause considerable visual morbidity. • Treatment of hypertension may resolve ocular features, but does not improve established vision loss. • Other vascular risk factors eg hyperglycaemia, dyslipidaemia, smoking and abnormal circulation compound the risk and effect of hypertension in the eye. Evidence base: common eye diseases are commoner if the patient is hypertensive • Cataract: In a meta-analysis of 25 studies across the world, risk of cataract was found to be increased in populations with hypertension independent of glycaemic risk, obesity or lipids [1]. We don’t know how generalisable this finding is. • Glaucoma: Nocturnal hypotension is also found to be associated with progression of visual field defects in glaucoma. • Late stage AMD: Some population studies show increased incidence with high © 2016 RNIB Reg charity nos 226227, SC039316 rnib.org.uk/gp [email protected] systolic BP [2], others show incidence associated with the metabolic syndrome but not specifically with hypertension [3]. • Other: Incidental retinal detachment in non-myopic eyes has been found to be more common [4] but again we don’t know how generalisable this is. -

Fabric Fiber Content
Fabric Types, Count & Fiber Content Zweigart Linen Count Content Belfast 32 100% linen Afghans - 100% Polyacrylic Cashel 28 100% linen Abby 18ct Alba 14ct Almanac 14ct Cork 19 100% linen Anne Cloth 18ct Baby Snuggle 18ct Country Home 18ct Dublin 25 100% linen Diamond 18ct Gloria 14ct Hearthside 14ct Edinborough 36 100% linen Honeycomb 18ct Novara 14ct Patrice 14ct Fine Linen 45 55% linen + 45% cotton Afghans - 100% Cotton Glasgow 28 100% linen Anne Cloth 18ct Augusta 14 ct Novara 14ct Kingston 50 100% linen Teresa 14ct Newcastle 40 100% linen Afghans- Misc Normandie 55% cotton + 45% linen Pastel LinenD 28 52% cotton + 48% linen Gloria 14ct 70% rayon + 30% linen Pearl Linen 20, 25, 28 60% polyester + 40% linen Merino 28ct 100% Wool Mosaik 18ct 52% cotton + 42% rayon Patterned Count Content Tannenbaum 18ct 52% cotton + 42% rayon Cottage Huck 14 100% cotton Aida Weave Count Content Belinda 20 52% cotton + 48% rayon Diana 20 52% cotton + 48% rayon Aida 8, 11, 14, 16, 18 100% cotton Newport 28 100% linen Country AidaD 7 100% polyacrylic Sambuca 28 60% polyester + 40% linen Damask Aida 11,14,18 52% cotton + 48% rayon Saronno 28 52% cotton + 48% rayon GoldauD 7 55% rayon + Shenandoah 28 55% linen + 45% rayon 40% cotton + 5% metallic Hardanger 22 100% cotton Canvas Count Content Hearthstone 14 60% cotton + 40% linen Congress 24 100% cotton Herta 6 100% cotton Congressa 24 100% cotton Huck 14 100% cotton Cordova 22 100% cotton Klostern 7 60% rayon + 40% cotton Double Mesh 5, 6.5, 7.5, 10, 12, Linen Hardanger 22 100% linen 14, 16, 18, 20 100% cotton -

Cotton Wool Spots the Moral of the Story Brown Et Al
Examining the Retina Assess the optic nerve Clinical Decisions in Retina What is the cup to disc ratio Is there good coloration and perfusion Mark T. Dunbar, O.D., F.A.A.O. Is it flat Choroidal or scleral crescent Mark Dunbar: Disclosure Examining the Retina Consultant for Allergan Pharmn What is the caliber of the retinal vessels Optometry Advisory Board for: Make sure you look and consciously take not of what the caliber is Allergan Carl Zeiss Meditec Narrowing of the vessels requires checking Alcon Nutritional the blood pressure Advisory Board Mark Dunbar does not own stock in any of the above companies Normal A/V ratio is 2/3, ¾ ArticDx What about the arterial light reflex? Examining the Retina Examining the Retina Don’t forget to look at the anterior vitreous The Macula Needs to be done on every dilated patient Is there a foveal light reflex (FLR)? Done at the slit lamp, looking posterior to Is it flat? the lens Is there any fluid, hemorrhage, or exudate Retroillumination may help if you suspect Presence of drusen vitreous cell RPE mottling More on this later Examining the Retina PVD The peripheral retina 65% of individuals > 65 have PVD It has to be done through a dilated pupil More common in women Don’t substitute imaging for indirect ophthalmoscopy More common following intraocular surgery Use Imaging as a compliment, but not substitute More common following Be systematic in your examination inflammation You should be able to see ora on “all” gazes More common in aphakes It’s all about technique -

Fabric Supplier List
FABRIC SUPPLIER LIST CANADA Kendor Textiles Ltd 1260 Cliveden Ave Delta BC V3M 6Y1 Canada 604.434.3233 [email protected] www.kendortextiles.com Fabrics Available: Fabric supplier. Eco-friendly. Organic. Knits: solids, prints, yarn dyes and warp. Wovens: solids and yarn dyes. End Use: activewear, bottomweights, medical, lingerie, childrenswear, swimwear, rainwear, skiwear and uniform. Natural & eco items include cottons, bamboo's, modals, linens, hemps, organic cottons & organic linens. Technical items include waterproof/breathable soft shells, antibacteric & wicking polyester & recycled polyesters. Is a proud representative of the British Millerain line of waxed cottons and wools, and are able to provide custom souring. Minimums: Carries stock. In-stock minimum: 5 yards/color. Minimum order for production: 10 yards/color. Gordon Fabrics LTD #1135-6900 Graybar Rd. Richmond BC Canada 604.275.2672 [email protected] Fabrics Available: Fabric Supplier. Importer. Jobber. Carries stock. Knits & Wovens: solids, prints, yarn dyes and novelties. End Use: activewear, borromweights, eveningwear/bridal, medical, lingerie and childrenswear. Minimums: In stock minimum 1 yard. Minimum order for production varies. StartUp Fashion Supplier List 2016 – Page 1 CHINA Ecopel (HX) Co., Ltd. China +86 216.767.9686 www.ecopel.cn Fabrics Available: Fake fur and leather garments. End Uses: Childrenswear, Menswear, Other, Womenswear. Minimums: Min. order 50-100 m Hangzhou New Design Source Textile Co., Ltd. China +86 057.182.530528 Fabrics Available: Knits, Polyester/Man-Made, Prints. End Uses: Juniors Fashion, Menswear, Womenswear. Minimums: Min order 50 m. Nantong Haukai Textile Co., Ltd. China +86 513.890.78626 www.huakaitex.com Fabrics Available: Cotton, Linen. End Uses: Corporatewear/Suiting, Menswear, Womenswear. -

Development of Novel Carbon Fiber Produced from Waste Fiber by Cabonization
Journal of Oleo Science Copyright ©2012 by Japan Oil Chemists’ Society J. Oleo Sci. 61, (10) 593-600 (2012) Development of Novel Carbon Fiber produced from Waste Fiber by Cabonization Naohito Kawasaki* , Hisato Tominaga, Fumihiko Ogata, Kenji Inoue and Moe Kankawa Faculty of Pharmacy, Kinki University, (3-4-1, Kowakae, Higashi-Osaka, Osaka 577-8502, JAPAN) Abstract: The volume of waste fiber has increased rapidly in recent years, and this trend is expected to continue. In this study, therefore, we attempted to convert waste fiber to carbonaceous materials by carbonization and investigated the basic properties of the resulting carbonized fibers. The results demonstrated that pores tend to form and specific surface areas change substantially, depending on the carbonization conditions. The carbonization conditions resulting in the largest specific surface areas included a temperature increase and retention times of 2 h. Carbonization temperatures resulting in the maximum values of 1000℃ were 900–1000℃ for wool and 1000℃ for both polyester and cotton. In particular, the specific surface area of cotton after carbonization at 1000℃ was 1253 m2/g, and scanning electron microscopy (SEM) micrographs showed that cotton retained its fibrous form after carbonization. Thus, it is possible to inexpensively convert waste fibers to carbonaceous material by carbonization. The results indicate that for cotton fiber in particular, the practical application of this process to the production of low-cost fibrous activated carbon would be possible, since cotton fiber retains its fibrous form under carbonization. Key words: Waste Fiber; Carbonization; Pore Size Distribution; Conversion 1 INTRODUCTION responsibility in their pursuit of profits. However, recycling In recent years, increases in population and industrial rates for fiber products have not been rising, largely activity have led to many mutually related problems world- because of consumer resistance to the use of recycled wide concerning the environment, resources, and energy. -

Eco-Friendly Dyeing of Wool and Pashmina Fabric Using Quercus Robur L. (Fruit Cups) Dye and Salix Alba L
AL SC R IEN TU C A E N F D O N U A N D D Journal of Applied and Natural Science 7 (1) : 138 – 143 (2015) A E I T L I O P JANS N P A ANSF 2008 Eco-friendly dyeing of wool and pashmina fabric using Quercus robur L. (fruit cups) dye and Salix alba L. (wood extract) mordant Syed Maqbool Geelani1*, Shoukat Ara1, Pradeep Kumar Mishra2, S.J.A. Bhat3, Syed Hanifa4, Shamsul Haq1, Iqbal Jeelani5, Gazala Qazi1, Asif H Sofi6, Shakeel A Mir5, P.A. Khan3, Sarfaraz A Wani6 and A S.M. Raja7 1Division of Environmental Sciences, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Shalimar-190025 (J&K), INDIA. 2Department of Chemical Engineering & Technology, Indian Institute of Technology (Banaras Hindu University), Varanasi-221005.(UP), INDIA 3Faculty of Forestry, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Wadura-193201, (J&K), INDIA 4Department of Food Sciences & Technology, University of Kashmir, Hazratbal, Srinagar-190006 (J&K), INDIA 5Division of Agricultural Statistics, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Shalimar-190025 (J&K), INDIA 6Division of Livestock Products Technology, Faculty of Veterinary Science & Animal Husbandry, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Shuhama-190006 (J&K), INDIA 7Division of Textile Manufacture and Textile chemistry, Central Sheep and wool Research Institute, Avikanagar - 304501 (Rajasthan), INDIA *Corresponding author. E-mail: [email protected] Received: November 24, 2014; Revised received: February 20, 2015; Accepted: March 19, 2015 Abstract: Study was conducted to investigate the dyeing potential of Quercus robur L. -

Sonicator Dyeing of Cotton, Wool and Silk with the Leaves Extract
Volume 6, Issue 1, Spring2009 Sonicator Dyeing of Cotton, Wool and Silk with the Leaves Extract Padma S Vankar*a, Rakhi Shanker a, Shalini Dixit a and Debajit Mahantab aFacility for Ecological and Analytical Testing Indian Institute of Technology, Kanpur-208 016, India bArunachal Pradesh State Council for Science & Technology Vivek-Vihar, Itanagar-791 113, Arunachal Pradesh ABSTRACT Malus sikkimensis. (Local name- Chap shaw sheng) belongs to family Rosaceae is being primarily used for preparing tea by Monpas tribes of Arunachal Pradesh. In the present study sonicator dyeing with Malus extract has been demonstrated. Pretreatment with 1-2 % metal mordant and using 5 % of plant extract (owf) was found to be optimum and showed very good hue colors for cotton, wool and silk dyed fabrics. We have demonstrated that the use of sonication in conjunction with metal mordanting has a synergistic effect on better dye uptake, dye adherence and eventually on good fastness properties of the dyed swatches of cotton, silk and wool. Keywords: Malus sikkimensis, natural dye, cotton, wool, silk, commercial dyeing 1. Introduction: Malus sikkimensis, belongs to family Rosaceae, is an evergreen woody tree with a maximum height of 40 m and diameter 2.3 m. It occurs in humid valley forest of Arunachal Pradesh. In the present study Malus leaves (figure-1) have been used as natural dye-stuff, which is primarily used as a substitute for tea by the local Arunachalees. The crude aqueous extract has been shown to dye cotton, silk and wool samples as shown in figures 5-7. Figure-1 Leaves of Malus sikkimensis Traditional Knowledge of its use: Stem and leaves of the plant locally known as 2.