Macaca Fuscata
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anatomical and Morphometric Study of Optic Foramen in North Indian Population
Published online: 26.06.2019 THIEME Original Article 53 Anatomical and Morphometric Study of Optic Foramen in North Indian Population Ajay Kumar1 Alok Tripathi1 Shilpi Jain1 Satyam Khare1 Ram Kumar Kaushik1 Hina Kausar1 Saurabh Arora1 1Department of Anatomy, Subharti Medical College, Swami Address for correspondence Alok Tripathi, MS, Department of Vivekanand Subharti, University, Meerut, Uttar Pradesh, India Anatomy, Subharti Medical College, Swami Vivekanand Subharti University, Meerut 250005, Uttar Pradesh, India (e-mail: [email protected]). Natl J Clin Anat 2019;8:53–56 Abstract Introduction Optic canal connects orbit to middle cranial fossa. Optic nerve and ophthalmic artery pass through this canal. The aim of the present study is to make morphometric and anatomical observations of endocranial opening of optic canal. Materials and Methods The observations were conducted on 30 dry adult human skulls. The observations were made on shape, margins, confluence, septations, dimensions, and distance of optic foramen from apex of petrous temporal bone. Result and Statistical Analysis On morphometric observation, transverse diameter (TD) was 6.00 mm and 6.15 mm on the left and the right side, respectively. The vertical diameter (VD) was 5.14 mm on the left side and 4.82 mm on the right side. The distance of optic foramen to apex of petrous temporal bone was 21.84 mm on the Keywords left side and 21.90 mm on the right side. The mean, standard deviation, range, and ► optic foramen p value were measured by using SPSS software version 19.00. ► dry skull Conclusion In the present study we attempt to provide a comprehensive anatomical ► morphometry and morphometric data of optic foramen that may help ophthalmologists and ► dimensions neurosurgeons during surgery. -

Septation of the Sphenoid Sinus and Its Clinical Significance
1793 International Journal of Collaborative Research on Internal Medicine & Public Health Septation of the Sphenoid Sinus and its Clinical Significance Eldan Kapur 1* , Adnan Kapidžić 2, Amela Kulenović 1, Lana Sarajlić 2, Adis Šahinović 2, Maida Šahinović 3 1 Department of anatomy, Medical faculty, University of Sarajevo, Čekaluša 90, 71000 Sarajevo, Bosnia and Herzegovina 2 Clinic for otorhinolaryngology, Clinical centre University of Sarajevo, Bolnička 25, 71000 Sarajevo, Bosnia and Herzegovina 3 Department of histology and embriology, Medical faculty, University of Sarajevo, Čekaluša 90, 71000 Sarajevo, Bosnia and Herzegovina * Corresponding Author: Eldan Kapur, MD, PhD Department of anatomy, Medical faculty, University of Sarajevo, Bosnia and Herzegovina Email: [email protected] Phone: 033 66 55 49; 033 22 64 78 (ext. 136) Abstract Introduction: Sphenoid sinus is located in the body of sphenoid, closed with a thin plate of bone tissue that separates it from the important structures such as the optic nerve, optic chiasm, cavernous sinus, pituitary gland, and internal carotid artery. It is divided by one or more vertical septa that are often asymmetric. Because of its location and the relationships with important neurovascular and glandular structures, sphenoid sinus represents a great diagnostic and therapeutic challenge. Aim: The aim of this study was to assess the septation of the sphenoid sinus and relationship between the number and position of septa and internal carotid artery in the adult BH population. Participants and Methods: A retrospective study of the CT analysis of the paranasal sinuses in 200 patients (104 male, 96 female) were performed using Siemens Somatom Art with the following parameters: 130 mAs: 120 kV, Slice: 3 mm. -

An Osteologic Study of Cranial Opening of Optic Canal in Gujarat Region
Original Article DOI: 10.7860/JCDR/2016/22110.8929 An Osteologic Study of Cranial Opening of Optic Canal in Section Gujarat Region Anatomy BINITA JIGNESHKUMAR PUROHIT1, PRAVEEN R SINGH2 ABSTRACT Similarly, morphologic features related with the canal were studied Introduction: Optic canal is a bony canal situated in between the by calculating frequency and proportions of various parameters. roots of lesser wings of sphenoid, lateral to body of sphenoid. Results: Optic canal was present in all 150 skulls studied bilaterally. It transmits optic nerve and ophthalmic artery, surrounded by The mean maximum dimension of the canal at cranial opening was meninges. Various authors have studied variations in skull foramina Keywords: ??????????????????????????????????5.03±0.72 mm on right side and 5.02±0.76 mm on left side. The and correlated clinically, as variants in the body structures have shape of the canal was ovoid at cranial opening in all the skulls been found to be associated with many inherited or acquired studied. Duplication of optic canal was present in one skull on left diseases. side. Recess was found in 105(35%) sides of total skulls observed. Aim: The present study aimed to examine morphologic and Fissure was found in 20(6.67%) sides and notch was observed in morphometric variations in cranial openings of optic canals. 30(10%) sides of total skulls. Materials and Methods: The study was undertaken in total 150 Conclusion: The optic canal showed variability in various dry adult human skulls. The variations in size, shape, presence or parameters. Knowledge regarding variations in size, shape and absence and duplication or multiplication if any, in optic canal were unusual features on cranial opening of optic canal can be helpful observed bilaterally. -

Anatomical Variations of Sphenoid Sinus: a Radiological Evaluation
ADDIS ABABA UNIVERSITY, COLLEGE OF HEALTH SCIENCES, SCHOOL OF MEDICINE, DEPARTMENT OF ANATOMY Anatomical Variations of Sphenoid Sinus: A Radiological Evaluation BY: Tizita Kinfe (BSc.) JUNE, 2019 Addis Ababa, Ethiopia Anatomical Variations of Sphenoid Sinus: A Radiological Evaluation Thesis submitted to Department of Anatomy, School of Medicine, College of Health Sciences, Addis Ababa University for the partial fulfillment of the requirements for the Degree of Masters of Science (MSc.) in Human Anatomy. By: Tizita Kinfe (BSc.) April, 2019 Addis Ababa, Ethiopia IDENTIFICATION Name of investigator: Tizita Kinfe (BSc.) Advisors: Principal Advisor: Abay Mulu (Ass. Prof. in Anatomy), Department of Anatomy, School of Medicine, College of Health Sciences, AAU Co advisors: 1. Dr Tequam Debebe (Ass. Prof. in Radiology, Consultant Neuroradiologist), Department of Radiology, School of Medicine, CHS, AAU. 2. Dr Seife Teferi (Aso. Prof. in Radiology, Radiation Physist), Department of Radiology, School of Medicine, CHS, AAU. Full title of project: Anatomic Variations of Sphenoid Sinus: A Radiological Evaluation Address of Investigator: AAU, College of Health Sciences, School of Medicine, Department of Anatomy Cell phone: +251-910603204 E-mail: [email protected] P.O. Box: 9086 Addis Ababa University School of Graduate Studies Declaration This is to certify that the thesis prepared by Tizita Kinfe, entitled, “Anatomic Variations of Sphenoid Sinus: A Radiological Evaluation” and submitted in partial fulfillment of the requirements for degree of Masters of Science in Anatomy complies with the regulation of the University and meets the accepted standards with respect to originality and quality. This thesis has not been presented for degree any other University, and that all sources of materials used for the thesis have been fully acknowledged. -

Dr. Hassna B. Jawad Cranial Cavity
Dr. Hassna B. Jawad Cranial cavity At the end of the lecture you should be able to: *Identify the anterior ,middle and posterior cranial fossa *Identify the foramen of the base of skull and the structures passed through it The inside view of cranium is known as cranial cavity. The cranial cavity is divided 2 parts: A. Calveria : lies superior and contains the following structures: -sulcus for superior sagittal sinus • -granular foveola • -arterial grooves • B. The base of the skull ( floor ) which consists of three fossae: 1. Anterior cranial fossa which accommodates the frontal lobe of brain. 2. Middle cranial fossa, much wider than the anterior cranial fossa contain the 2 temporal lobes of brain. 3. Posterior cranial fossa is much shallower and wider than the middle cranial fossa and it accommodates the cerebellum. .1 Anterior Cranial Fossa: .2 Is a depression in the floor of the cranial vault which houses the projecting frontal lobes of the brain. It is formed by the following bones: 1.Orbital plates of the frontal bone. .3 2.The cribriform plate of ethmoid bone. .4 3.The lesser wings and the front of the body of sphenoid bone. .5 1 Dr. Hassna B. Jawad Boundaries: .6 1.Anteriorly and laterally by the inner surface of the frontal bone. .7 In the midline there is a crest for the attachment of the falx cerebri. .8 2.Posteriorly is formed by the lesser wing of the sphenoid bone with anterior clinoid process and .9 the groove of optic chiasma. The middle part of anterior cranial fossa is limited posteriorly by the groove for the optic .11 chiasma. -
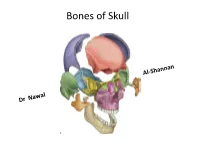
Norma Lateralis: Lateral View of the Skull • Bones; • 1.Temporal Bone • 2.Temporal Lines • 3Zygomatic Arch • 4.External Auditory Meatus • 5
Bones of Skull Views of the skull Each aspect of skull is called Norma : study from above is called Norma verticalis Below : Norma - basalis Behind : Norma - occipitalis Front : Norma - frontalis Side to side: Norma - lateralis Norma verticalis = Vault = arched roof = dome • Bones: • Frontal • Parietal • Small part of occipital • Vertex : the highest point of the sagittal suture • Paraietal foramen – 3.5 cm infront of lambda • Bregma: cranial point between coronal and sagittal suture • Lambda: cranial point at the junction of lambda and sagittal suture • Paraietal eminence: maximum convex part of parietal bone Vault Norma occipitalis: posterior aspect of the skull • Bones: • 1. Posterior part of parietal bone • 2.mastoid process of temporal bone • 3.squamous part of occipital bone • Sutures: • 1.lambdoid • Paraietomastoid • occipitomastoid • External occipital protuberance • Superior and highest nuchal line Posterior View &Sutures Norma Frontalis • Can be divided into upper and lower Upper part: Super ciliary arches: curved elevations above the supraorbital margins Glabella: median elevation between the two supraciliary arches Nasion: median point at the root of the nose oribital opening : supraorbital margin formed by frontal bone supraorbital notch or foramen transmit supraorbital vessels and nerves Infra orbital margin formed by maxilla medially and zygomatic bone laterally Medial margin: formed by frontal bone above and anterior lacrimal crest of the maxilla below Lateral margin; formed by frontal process of zygomatic bone and zygomatic process of frontal bone Anterior nasal aperture Nasal bones Norma frontalis Upper part / Orbit Upper part Norma frontalis lower part of the face: Maxilla have processess Frontal process of maxilla Zygomatic process Alveolar process Zygomatic bones Body ,processes ,arch Zygomaticofacial foramen Z-f nerve Mandible : mental f -mental n and v Lower part Norma lateralis: lateral view of the skull • Bones; • 1.temporal bone • 2.temporal lines • 3Zygomatic arch • 4.External auditory meatus • 5. -

A Study on Ossified Carotico-Clinoid Ligament in Human Skulls in Rayalaseema Zone
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 19, Issue 1 Ser.6 (January. 2020), PP 30-33 www.iosrjournals.org A Study on Ossified Carotico-Clinoid Ligament in Human Skulls in Rayalaseema Zone 1.Dr.K.Prathiba, 2.*Dr.M.K. Lalitha Kumari, 3. Dr.C.Sreekanth, 4. Dr.D.Srivani 1.Associate Professor,Dept. Of Anatomy,SPMC (W),SVIMS, Tirupati, A.P 2*.Tutor, Dept. Of Anatomy,SPMC (W),SVIMS, Tirupati, A.P, 3. Associate Professor,Dept. Of Anatomy,SPMC (W),SVIMS, Tirupati, A.P 4. Assistant Professor,Dept. Of Anatomy,SPMC (W),SVIMS, Tirupati, A.P Corresponding Author:** Dr.M.K Lalitha Kumari Abstract: Introduction: Anomalous presence or absence, agenesis or multiplications of these foramina’s are of interest in human skulls, in order to achieve better comprehension of neurovascular content through them. Ligaments bridging the notches sometimes ossify which may lead to compression of the structures passing through foramina’s thereby they may have significant clinical signs and symptoms. Presence of carotico-clinoid foramen is the result of ossification of either carotico-clinoid ligament or of dural fold extending between anterior and middle clinoid processes of sphenoid bone. Materials and methods: The study was done in 50 adult dry human skulls collected from Sri Padmavathi Medical College for Women, SVIMS, Tirupati and S.V. Medical college and S.V University (Anthropology department). Results: In 100 adult dry human skulls, 12 skulls of unknown sex showed “Ossified carotico-clinoid ligament” out of which 7 were on the left side and 5 were on right. -

Computed Tomographic Study of Sphenoid Sinus and Its Septations
Indian Journal of Clinical Anatomy and Physiology 2019;6(3):325–330 Content available at: iponlinejournal.com Indian Journal of Clinical Anatomy and Physiology Journal homepage: www.innovativepublication.com Original Research Article Computed tomographic study of sphenoid sinus and its septations Kuldeep Kumar1, Pooja Gautam2,*, A P Mishra3, C S Ramesh Babu4 1Dept. of Anatomy, Government Medical College, Kannauj, Uttar Pradesh, India 2Dept. of Anatomy, Autonomous State Medical College, Basti, Uttar Pradesh, India 3Dept. of Radiology, Rama Mediacal University, Uttar Pradesh, India 4Dept. of Anatomy, Muzaffar Nagar Medical College, Muzaffarnagar, Uttar Pradesh, India ARTICLEINFO ABSTRACT Article history: Introduction: Knowledge of sphenoid sinus anatomy and its variations is of prime importance in trans- Received 02-07-2019 sphenoidal endoscopic skull base surgery. Accepted 21-08-2019 Aim: The aim of the present study was to measure the bilateral sphenoidal sinus parameters [depth, width, Available online 12-10-2019 and height] and determine the number and pattern of attachments of intra-sphenoid sinus septations in 52 subjects by computed tomography. Materials and Methods: Cranial computed tomographic images of 52 normal subjects were included Keywords: in our study. Four cases in whom the septations were absent were excluded from the measurement Sphenoid sinus of dimensions of the sinus. The depth, width, and height in 48 sphenoidal sinuses were measured Morphometric study electronically. The coronal and axial sections were taken and subsequently examined. Pattern Results: The mean depth, width, and height of sphenoid sinus in males on right side are 2.2458cm, Septations 1.555cm, 1.9044 cm and on left side are 2.3877cm, 1.6519cm, 1.8613cm and in females on right side are 2.0475cm, 1.6258cm,2.05cm and on left side are 2.066cm, 1.2891cm and 1.7941 cm respectively. -

Sphenoid Sinus Anatomical Relations and Their Implications In
Available online at www.ijmrhs.com cal R edi ese M ar of c l h a & n r H u e o a J l l t h International Journal of Medical Research & a S n ISSN No: 2319-5886 o c i t i Health Sciences, 2017, 6(9): 162-166 e a n n c r e e t s n I • • IJ M R H S Sphenoid Sinus Anatomical Relations and their Implications in Endoscopic Sinus Surgery Mubina Lakhani1*, Madeeha Sadiq2 and Sehrish Mukhtar3 1Senior Lecturer, Department of Anatomy, Ziauddin University, Karachi, Pakistan 2Assistant Professor, Department of Anatomy, Ziauddin University, Karachi, Pakistan 3Assistant Professor, Department of Anatomy, Jinnah Medical and Dental College, Karachi, Pakistan *Corresponding e-mail: [email protected] ABSTRACT With advances in endoscopic sinus surgery (ESS), radiologist and otolaryngologists should have thorough knowledge of anatomy of paranasal sinus (PNS). Regarding this, sphenoid sinuses are the most variable among paranasal sinuses. the anatomical relationship of crucial neurovascular structures for example internal carotid artery (ICA) and optic nerve (ON) is extremely variable and these structures are at a risk during ESS. This article will help readers understand the relationship of neurovascular structures with sphenoid sinus (SS) more precisely. Keywords: Endoscopic sinus surgery, Sphenoid sinus, Internal carotid artery, Optic nerve, Computed tomography INTRODUCTION Interest of surgeons in both the anatomy and pathophysiology of the PNS has been stimulated due to advances in ESS. The ultimate aim of surgeon is aerating the sinuses and restoring mucociliary clearance in order to restore the function of paranasal sinuses [1]. -

Imaging of Skull Base
MINI-SYMPOSIA-HEAD AND NECK Imaging of skull base: Pictorial essay Abhijit A Raut, Prashant S Naphade1, Ashish Chawla2 Department of Radiology, Seven Hills Hospital, Mumbai, 1Department of Radiology, Employee’s State Insurance Corporation Hospital, Mumbai, Maharashtra, 2Department of Radiology, Sri Aurobindo Medical College and Postgraduate Institute, Indore, Madhya Pradesh, India Correspondence: Dr. Abhijit Raut, Department of Radiology, Seven Hills Hospital, Marol Maroshi Road, Andheri East, Mumbai ‑ 400 059, India. E‑mail: [email protected] Abstract The skull base anatomy is complex. Numerous vital neurovascular structures pass through multiple channels and foramina located in the base skull. With the advent of computerized tomography (CT) and magnetic resonance imaging (MRI), accurate preoperative lesion localization and evaluation of its relationship with adjacent neurovascular structures is possible. It is imperative that the radiologist and skull base surgeons are familiar with this complex anatomy for localizing the skull base lesion, reaching appropriate differential diagnosis, and deciding the optimal surgical approach. CT and MRI are complementary to each other and are often used together for the demonstration of the full disease extent. This article focuses on the radiological anatomy of the skull base and discusses few of the common pathologies affecting the skull base. Key words: Computed tomography; magnetic resonance imaging; skull base Introduction The skull base is composed of five bones: (1) ethmoid, (2) sphenoid, (3) occipital, (4) paired temporal, and (5) paired The skull base forms the floor of the cranial cavity that frontal bones. Three naturally contoured regions can be separates brain from facial structures and suprahyoid identified when skull base is viewed from above [Figure 1]. -

Bridges of the Sella Turcica — Anatomy and Topography
FOLIA MEDICA CRACOVIENSIA 97 Vol. LII, 3–4, 2012: 97–101 PL ISSN 0015-5616 JANUSZ SKRZAT1, Izabela Mróz1, JUSTYNA MARCHEWKA1,2 BRIDGES OF THE SELLA TURCICA — ANATOMY AND TOPOGRAPHY Abstract: Bridges of the sella turcica — anatomy and topography This paper presents anatomy and topography of the inconstant osseous bridges that may occur in the sella turcica region. The interclinoid bridge and the caroticoclinoid bridge can be formed in con- sequence of abnormal ossification of the dural folds or disturbances in development of the sphenoid bone. Their presence may be of clinical importance because of potential influence on the neurovascular structures passing in the vicinity of the clinoid processes of the sphenoid bone. Key words: sellar bridge, sella turcica, sphenoid bone INTRODUCTION Process of ossification of cranial structures might be a natural consequence of ageing or a result of adaptation changes of the axial skeleton, although sometimes it is difficult to guess what are the real causative factors [1–3]. The folds of the dura mater (ligaments) that are attached to the clinoid processes (the anterior, the middle and the posterior) may occasionally ossify and form bony bridges of the sphenoid bone. These inconstant osseous structures may also derive from the cartilaginous tissue [4, 5]. The formation of the osseous bridges within the sellar region may also effect of disturbances in development of the sphenoid bone [4, 6]. The ligament between anterior and posterior clinoid process is known as the interclinoid ligament, and the bony connection between these processes is known as the interclinoid bridge. In turn, the anterior and middle clinoid processes may be connected by the caroticoclinoid ligament which may ossify forming a caroticoclinoid bridge. -

Unilateral Duplication of Optic Canal: a Case Report
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 13, Issue 4 Ver. V. (Apr. 2014), PP 82-83 www.iosrjournals.org Unilateral Duplication of Optic Canal: A Case Report Vanitha1, Taqdees Fatima2,H. S. Kadlimatti3 1(Department of Anatomy, ESIC Medical College Gulbarga/ Rajiv Gandhi university of health Sciences, Karnataka, India) 2(Department of Anatomy, ESIC Medical College Gulbarga/ Rajiv Gandhi university of health Sciences, Karnataka, India) 3(Department of Anatomy, ESIC Medical College Gulbarga/ Rajiv Gandhi university of health Sciences, Karnataka, India) Abstract: Optic canal is formed by two roots of lesserwing and body of sphenoid bone. It transmits optic nerve, ophthalmic artery and periarterial sympathetic plexus. During demonstration classes for undergraduate students we observed optic canal of left orbit of the adult skull, showing a thin spicule of bone which divided optic canal into two. Unilateral presence of duplicated optic canal is very rare. This case report may be useful for surgeons and radiologists. Keywords: Duplication, duramater, optic canal, ossification, I. Introduction Optic canal is formed by the two roots of lesser wing and body of sphenoid bone. Anterior root of lesser wing of sphenoid is flat and posterior root is thick, triangular. The optic canal lies between these roots and connects the orbit to the middle cranial fossa. It is traversed by the optic nerve and its meningeal coverings, the ophthalmic artery and by the branches from the periarterial sympathetic plexus. The artery and nerve are separated by a layer of dense connective tissue which may occasionally be ossified to result in the duplication of the optic canal.