Development of the Skull
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anatomical and Morphometric Study of Optic Foramen in North Indian Population
Published online: 26.06.2019 THIEME Original Article 53 Anatomical and Morphometric Study of Optic Foramen in North Indian Population Ajay Kumar1 Alok Tripathi1 Shilpi Jain1 Satyam Khare1 Ram Kumar Kaushik1 Hina Kausar1 Saurabh Arora1 1Department of Anatomy, Subharti Medical College, Swami Address for correspondence Alok Tripathi, MS, Department of Vivekanand Subharti, University, Meerut, Uttar Pradesh, India Anatomy, Subharti Medical College, Swami Vivekanand Subharti University, Meerut 250005, Uttar Pradesh, India (e-mail: [email protected]). Natl J Clin Anat 2019;8:53–56 Abstract Introduction Optic canal connects orbit to middle cranial fossa. Optic nerve and ophthalmic artery pass through this canal. The aim of the present study is to make morphometric and anatomical observations of endocranial opening of optic canal. Materials and Methods The observations were conducted on 30 dry adult human skulls. The observations were made on shape, margins, confluence, septations, dimensions, and distance of optic foramen from apex of petrous temporal bone. Result and Statistical Analysis On morphometric observation, transverse diameter (TD) was 6.00 mm and 6.15 mm on the left and the right side, respectively. The vertical diameter (VD) was 5.14 mm on the left side and 4.82 mm on the right side. The distance of optic foramen to apex of petrous temporal bone was 21.84 mm on the Keywords left side and 21.90 mm on the right side. The mean, standard deviation, range, and ► optic foramen p value were measured by using SPSS software version 19.00. ► dry skull Conclusion In the present study we attempt to provide a comprehensive anatomical ► morphometry and morphometric data of optic foramen that may help ophthalmologists and ► dimensions neurosurgeons during surgery. -

Septation of the Sphenoid Sinus and Its Clinical Significance
1793 International Journal of Collaborative Research on Internal Medicine & Public Health Septation of the Sphenoid Sinus and its Clinical Significance Eldan Kapur 1* , Adnan Kapidžić 2, Amela Kulenović 1, Lana Sarajlić 2, Adis Šahinović 2, Maida Šahinović 3 1 Department of anatomy, Medical faculty, University of Sarajevo, Čekaluša 90, 71000 Sarajevo, Bosnia and Herzegovina 2 Clinic for otorhinolaryngology, Clinical centre University of Sarajevo, Bolnička 25, 71000 Sarajevo, Bosnia and Herzegovina 3 Department of histology and embriology, Medical faculty, University of Sarajevo, Čekaluša 90, 71000 Sarajevo, Bosnia and Herzegovina * Corresponding Author: Eldan Kapur, MD, PhD Department of anatomy, Medical faculty, University of Sarajevo, Bosnia and Herzegovina Email: [email protected] Phone: 033 66 55 49; 033 22 64 78 (ext. 136) Abstract Introduction: Sphenoid sinus is located in the body of sphenoid, closed with a thin plate of bone tissue that separates it from the important structures such as the optic nerve, optic chiasm, cavernous sinus, pituitary gland, and internal carotid artery. It is divided by one or more vertical septa that are often asymmetric. Because of its location and the relationships with important neurovascular and glandular structures, sphenoid sinus represents a great diagnostic and therapeutic challenge. Aim: The aim of this study was to assess the septation of the sphenoid sinus and relationship between the number and position of septa and internal carotid artery in the adult BH population. Participants and Methods: A retrospective study of the CT analysis of the paranasal sinuses in 200 patients (104 male, 96 female) were performed using Siemens Somatom Art with the following parameters: 130 mAs: 120 kV, Slice: 3 mm. -

An Osteologic Study of Cranial Opening of Optic Canal in Gujarat Region
Original Article DOI: 10.7860/JCDR/2016/22110.8929 An Osteologic Study of Cranial Opening of Optic Canal in Section Gujarat Region Anatomy BINITA JIGNESHKUMAR PUROHIT1, PRAVEEN R SINGH2 ABSTRACT Similarly, morphologic features related with the canal were studied Introduction: Optic canal is a bony canal situated in between the by calculating frequency and proportions of various parameters. roots of lesser wings of sphenoid, lateral to body of sphenoid. Results: Optic canal was present in all 150 skulls studied bilaterally. It transmits optic nerve and ophthalmic artery, surrounded by The mean maximum dimension of the canal at cranial opening was meninges. Various authors have studied variations in skull foramina Keywords: ??????????????????????????????????5.03±0.72 mm on right side and 5.02±0.76 mm on left side. The and correlated clinically, as variants in the body structures have shape of the canal was ovoid at cranial opening in all the skulls been found to be associated with many inherited or acquired studied. Duplication of optic canal was present in one skull on left diseases. side. Recess was found in 105(35%) sides of total skulls observed. Aim: The present study aimed to examine morphologic and Fissure was found in 20(6.67%) sides and notch was observed in morphometric variations in cranial openings of optic canals. 30(10%) sides of total skulls. Materials and Methods: The study was undertaken in total 150 Conclusion: The optic canal showed variability in various dry adult human skulls. The variations in size, shape, presence or parameters. Knowledge regarding variations in size, shape and absence and duplication or multiplication if any, in optic canal were unusual features on cranial opening of optic canal can be helpful observed bilaterally. -

Anatomical Variations of Sphenoid Sinus: a Radiological Evaluation
ADDIS ABABA UNIVERSITY, COLLEGE OF HEALTH SCIENCES, SCHOOL OF MEDICINE, DEPARTMENT OF ANATOMY Anatomical Variations of Sphenoid Sinus: A Radiological Evaluation BY: Tizita Kinfe (BSc.) JUNE, 2019 Addis Ababa, Ethiopia Anatomical Variations of Sphenoid Sinus: A Radiological Evaluation Thesis submitted to Department of Anatomy, School of Medicine, College of Health Sciences, Addis Ababa University for the partial fulfillment of the requirements for the Degree of Masters of Science (MSc.) in Human Anatomy. By: Tizita Kinfe (BSc.) April, 2019 Addis Ababa, Ethiopia IDENTIFICATION Name of investigator: Tizita Kinfe (BSc.) Advisors: Principal Advisor: Abay Mulu (Ass. Prof. in Anatomy), Department of Anatomy, School of Medicine, College of Health Sciences, AAU Co advisors: 1. Dr Tequam Debebe (Ass. Prof. in Radiology, Consultant Neuroradiologist), Department of Radiology, School of Medicine, CHS, AAU. 2. Dr Seife Teferi (Aso. Prof. in Radiology, Radiation Physist), Department of Radiology, School of Medicine, CHS, AAU. Full title of project: Anatomic Variations of Sphenoid Sinus: A Radiological Evaluation Address of Investigator: AAU, College of Health Sciences, School of Medicine, Department of Anatomy Cell phone: +251-910603204 E-mail: [email protected] P.O. Box: 9086 Addis Ababa University School of Graduate Studies Declaration This is to certify that the thesis prepared by Tizita Kinfe, entitled, “Anatomic Variations of Sphenoid Sinus: A Radiological Evaluation” and submitted in partial fulfillment of the requirements for degree of Masters of Science in Anatomy complies with the regulation of the University and meets the accepted standards with respect to originality and quality. This thesis has not been presented for degree any other University, and that all sources of materials used for the thesis have been fully acknowledged. -

Dr. Hassna B. Jawad Cranial Cavity
Dr. Hassna B. Jawad Cranial cavity At the end of the lecture you should be able to: *Identify the anterior ,middle and posterior cranial fossa *Identify the foramen of the base of skull and the structures passed through it The inside view of cranium is known as cranial cavity. The cranial cavity is divided 2 parts: A. Calveria : lies superior and contains the following structures: -sulcus for superior sagittal sinus • -granular foveola • -arterial grooves • B. The base of the skull ( floor ) which consists of three fossae: 1. Anterior cranial fossa which accommodates the frontal lobe of brain. 2. Middle cranial fossa, much wider than the anterior cranial fossa contain the 2 temporal lobes of brain. 3. Posterior cranial fossa is much shallower and wider than the middle cranial fossa and it accommodates the cerebellum. .1 Anterior Cranial Fossa: .2 Is a depression in the floor of the cranial vault which houses the projecting frontal lobes of the brain. It is formed by the following bones: 1.Orbital plates of the frontal bone. .3 2.The cribriform plate of ethmoid bone. .4 3.The lesser wings and the front of the body of sphenoid bone. .5 1 Dr. Hassna B. Jawad Boundaries: .6 1.Anteriorly and laterally by the inner surface of the frontal bone. .7 In the midline there is a crest for the attachment of the falx cerebri. .8 2.Posteriorly is formed by the lesser wing of the sphenoid bone with anterior clinoid process and .9 the groove of optic chiasma. The middle part of anterior cranial fossa is limited posteriorly by the groove for the optic .11 chiasma. -

The Morphology and Biomechanics of Jaw Structures in Chondrichthyes
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 2013 THE MORPHOLOGY AND BIOMECHANICS OF JAW STRUCTURES IN CHONDRICHTHYES Jordan Balaban University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Balaban, Jordan, "THE MORPHOLOGY AND BIOMECHANICS OF JAW STRUCTURES IN CHONDRICHTHYES" (2013). Open Access Master's Theses. Paper 130. https://digitalcommons.uri.edu/theses/130 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. THE MORPHOLOGY AND BIOMECHANICS OF JAW STRUCTURES IN CHONDRICHTHYES BY JORDAN BALABAN A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN BIOLOGICAL AND ENVIRONMENTAL SCIENCES UNIVERSITY OF RHODE ISLAND 2013 MASTER OF SCIENCE THESIS OF JORDAN BALABAN APPROVED: Thesis Committee: Major Professor____Dr. Cheryl Wilga________________ ____Dr. Adam P. Summers____________ _____Dr. Holly Dunsworth_____________ ____Dr. Nasser H. Zawia______________ DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2013 ABSTRACT The skeletons of chondrichthyans (sharks, skates, rays, and chimeras) are composed entirely of cartilage, yet must still provide the skeletal support that bone does in other vertebrates. There is also an incredible range of diversity in the morphology of the cartilaginous skeleton of the feeding apparatus in Chondrichthyans. The goal of this research is to provide insight into the morphological evolution and biomechanical function of the cranial skeleton in chondrichthyans. Feeding style changes can occur with morphological changes in the skeletal elements of the shark feeding apparatus. -
Norma Lateralis: Lateral View of the Skull • Bones; • 1.Temporal Bone • 2.Temporal Lines • 3Zygomatic Arch • 4.External Auditory Meatus • 5
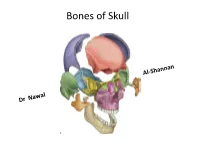
Bones of Skull Views of the skull Each aspect of skull is called Norma : study from above is called Norma verticalis Below : Norma - basalis Behind : Norma - occipitalis Front : Norma - frontalis Side to side: Norma - lateralis Norma verticalis = Vault = arched roof = dome • Bones: • Frontal • Parietal • Small part of occipital • Vertex : the highest point of the sagittal suture • Paraietal foramen – 3.5 cm infront of lambda • Bregma: cranial point between coronal and sagittal suture • Lambda: cranial point at the junction of lambda and sagittal suture • Paraietal eminence: maximum convex part of parietal bone Vault Norma occipitalis: posterior aspect of the skull • Bones: • 1. Posterior part of parietal bone • 2.mastoid process of temporal bone • 3.squamous part of occipital bone • Sutures: • 1.lambdoid • Paraietomastoid • occipitomastoid • External occipital protuberance • Superior and highest nuchal line Posterior View &Sutures Norma Frontalis • Can be divided into upper and lower Upper part: Super ciliary arches: curved elevations above the supraorbital margins Glabella: median elevation between the two supraciliary arches Nasion: median point at the root of the nose oribital opening : supraorbital margin formed by frontal bone supraorbital notch or foramen transmit supraorbital vessels and nerves Infra orbital margin formed by maxilla medially and zygomatic bone laterally Medial margin: formed by frontal bone above and anterior lacrimal crest of the maxilla below Lateral margin; formed by frontal process of zygomatic bone and zygomatic process of frontal bone Anterior nasal aperture Nasal bones Norma frontalis Upper part / Orbit Upper part Norma frontalis lower part of the face: Maxilla have processess Frontal process of maxilla Zygomatic process Alveolar process Zygomatic bones Body ,processes ,arch Zygomaticofacial foramen Z-f nerve Mandible : mental f -mental n and v Lower part Norma lateralis: lateral view of the skull • Bones; • 1.temporal bone • 2.temporal lines • 3Zygomatic arch • 4.External auditory meatus • 5. -

A Study on Ossified Carotico-Clinoid Ligament in Human Skulls in Rayalaseema Zone
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 19, Issue 1 Ser.6 (January. 2020), PP 30-33 www.iosrjournals.org A Study on Ossified Carotico-Clinoid Ligament in Human Skulls in Rayalaseema Zone 1.Dr.K.Prathiba, 2.*Dr.M.K. Lalitha Kumari, 3. Dr.C.Sreekanth, 4. Dr.D.Srivani 1.Associate Professor,Dept. Of Anatomy,SPMC (W),SVIMS, Tirupati, A.P 2*.Tutor, Dept. Of Anatomy,SPMC (W),SVIMS, Tirupati, A.P, 3. Associate Professor,Dept. Of Anatomy,SPMC (W),SVIMS, Tirupati, A.P 4. Assistant Professor,Dept. Of Anatomy,SPMC (W),SVIMS, Tirupati, A.P Corresponding Author:** Dr.M.K Lalitha Kumari Abstract: Introduction: Anomalous presence or absence, agenesis or multiplications of these foramina’s are of interest in human skulls, in order to achieve better comprehension of neurovascular content through them. Ligaments bridging the notches sometimes ossify which may lead to compression of the structures passing through foramina’s thereby they may have significant clinical signs and symptoms. Presence of carotico-clinoid foramen is the result of ossification of either carotico-clinoid ligament or of dural fold extending between anterior and middle clinoid processes of sphenoid bone. Materials and methods: The study was done in 50 adult dry human skulls collected from Sri Padmavathi Medical College for Women, SVIMS, Tirupati and S.V. Medical college and S.V University (Anthropology department). Results: In 100 adult dry human skulls, 12 skulls of unknown sex showed “Ossified carotico-clinoid ligament” out of which 7 were on the left side and 5 were on right. -

A Partial Braincase and Other Skeletal Remains of Oligocene Angel Sharks (Chondrichthyes, Squatiniformes) from Northwest Belgium, with Comments on Squatinoid Taxonomy
Contributions to Zoology, 85 (2) 147-171 (2016) A partial braincase and other skeletal remains of Oligocene angel sharks (Chondrichthyes, Squatiniformes) from northwest Belgium, with comments on squatinoid taxonomy Frederik H. Mollen1, 5, Barry W.M. van Bakel2, 3, John W.M. Jagt4 1 Elasmobranch Research, Rehaegenstraat 4, 2820 Bonheiden, Belgium 2 Oertijdmuseum De Groene Poort, Bosscheweg 80, 5283 WB Boxtel, The Netherlands 3 Naturalis Biodiversity Center, P.O. Box 9517, 2300 RA Leiden, The Netherlands 4 Natuurhistorisch Museum Maastricht, de Bosquetplein 6-7, 6211 KJ Maastricht, The Netherlands 5 E-mail: [email protected] Key words: chondrocranium, CT scanning, neurocranium, Pristiophorus, Squatina, vertebrae Abstract Orbital region (i.e., orbito-temporal or sphenoid region) ....................................................................................... 151 A detailed redescription of a chondrocranium from the basal Otic region (i.e., labyrinth or auditory region) ............... 152 Boom Clay Formation (Rupelian, Upper Oligocene) at the SVK Occipital region ...................................................................... 155 clay pit, Sint-Niklaas (province of Oost-Vlaanderen, Belgium), Results ............................................................................................. 157 previously assigned to the sawshark Pristiophorus rupeliensis, is Re-identification of chondrocranium ................................ 157 presented. The chondrocranium is re-identified as that of an an- Other angel shark skeletal -

Computed Tomographic Study of Sphenoid Sinus and Its Septations
Indian Journal of Clinical Anatomy and Physiology 2019;6(3):325–330 Content available at: iponlinejournal.com Indian Journal of Clinical Anatomy and Physiology Journal homepage: www.innovativepublication.com Original Research Article Computed tomographic study of sphenoid sinus and its septations Kuldeep Kumar1, Pooja Gautam2,*, A P Mishra3, C S Ramesh Babu4 1Dept. of Anatomy, Government Medical College, Kannauj, Uttar Pradesh, India 2Dept. of Anatomy, Autonomous State Medical College, Basti, Uttar Pradesh, India 3Dept. of Radiology, Rama Mediacal University, Uttar Pradesh, India 4Dept. of Anatomy, Muzaffar Nagar Medical College, Muzaffarnagar, Uttar Pradesh, India ARTICLEINFO ABSTRACT Article history: Introduction: Knowledge of sphenoid sinus anatomy and its variations is of prime importance in trans- Received 02-07-2019 sphenoidal endoscopic skull base surgery. Accepted 21-08-2019 Aim: The aim of the present study was to measure the bilateral sphenoidal sinus parameters [depth, width, Available online 12-10-2019 and height] and determine the number and pattern of attachments of intra-sphenoid sinus septations in 52 subjects by computed tomography. Materials and Methods: Cranial computed tomographic images of 52 normal subjects were included Keywords: in our study. Four cases in whom the septations were absent were excluded from the measurement Sphenoid sinus of dimensions of the sinus. The depth, width, and height in 48 sphenoidal sinuses were measured Morphometric study electronically. The coronal and axial sections were taken and subsequently examined. Pattern Results: The mean depth, width, and height of sphenoid sinus in males on right side are 2.2458cm, Septations 1.555cm, 1.9044 cm and on left side are 2.3877cm, 1.6519cm, 1.8613cm and in females on right side are 2.0475cm, 1.6258cm,2.05cm and on left side are 2.066cm, 1.2891cm and 1.7941 cm respectively. -

Connecting the Chondrocranium: Biomechanics of the Suspensorium in Reptiles
70 (3): 275 – 290 © Senckenberg Gesellschaft für Naturforschung, 2020. 2020 VIRTUAL ISSUE on Recent Advances in Chondrocranium Research | Guest Editor: Ingmar Werneburg Connecting the chondrocranium: Biomechanics of the suspensorium in reptiles Alec T. Wilken 1, *, Kaleb C. Sellers 1, Ian N. Cost 2, Rachel E. Rozin 1, Kevin M. Middleton 1 & Casey M. Holliday 1 1 Department of Pathology and Anatomical Sciences, University of Missouri, M263, Medical Sciences Building, Columbia, MO, 65212, USA — 2 Department of Biology, Albright College, 13th and Bern Streets, Reading, PA, 19612, USA — * Corresponding author; atwxb6@ mail.missouri.edu Submitted February 07, 2020. Accepted June 8, 2020. Published online at www.senckenberg.de/vertebrate-zoology on June 16, 2020. Published in print on Q3/2020. Editor in charge: Ingmar Werneburg Abstract Gnathostomes all share the common challenge of assembling 1st pharyngeal arch elements and associated dermal bones (suspensorium) with the neurocranium into a functioning linkage system. In many tetrapods, the otic and palatobasal articulations between suspensorium and neurocranial elements form the joints integral for cranial kinesis. Among sauropsids, the otic (quadratosquamosal) joint is a key feature in this linkage system and shows considerable variability in shape, tissue-level construction and mobility among lineages of reptiles. Here we explore the biomechanics of the suspensorium and the otic joint in fve disparate species of sauropsids of different kinetic capacity (two squamates, one non-avian theropod dinosaur, and two avian species). Using 3D muscle modeling, comparisons of muscle moments, joint surface areas, cross-sectional geometries, and fnite element analysis, we characterize biomechanical differences in the resultants of protractor muscles, loading of otic joints, and bending properties of pterygoid bones. -

Sphenoid Sinus Anatomical Relations and Their Implications In
Available online at www.ijmrhs.com cal R edi ese M ar of c l h a & n r H u e o a J l l t h International Journal of Medical Research & a S n ISSN No: 2319-5886 o c i t i Health Sciences, 2017, 6(9): 162-166 e a n n c r e e t s n I • • IJ M R H S Sphenoid Sinus Anatomical Relations and their Implications in Endoscopic Sinus Surgery Mubina Lakhani1*, Madeeha Sadiq2 and Sehrish Mukhtar3 1Senior Lecturer, Department of Anatomy, Ziauddin University, Karachi, Pakistan 2Assistant Professor, Department of Anatomy, Ziauddin University, Karachi, Pakistan 3Assistant Professor, Department of Anatomy, Jinnah Medical and Dental College, Karachi, Pakistan *Corresponding e-mail: [email protected] ABSTRACT With advances in endoscopic sinus surgery (ESS), radiologist and otolaryngologists should have thorough knowledge of anatomy of paranasal sinus (PNS). Regarding this, sphenoid sinuses are the most variable among paranasal sinuses. the anatomical relationship of crucial neurovascular structures for example internal carotid artery (ICA) and optic nerve (ON) is extremely variable and these structures are at a risk during ESS. This article will help readers understand the relationship of neurovascular structures with sphenoid sinus (SS) more precisely. Keywords: Endoscopic sinus surgery, Sphenoid sinus, Internal carotid artery, Optic nerve, Computed tomography INTRODUCTION Interest of surgeons in both the anatomy and pathophysiology of the PNS has been stimulated due to advances in ESS. The ultimate aim of surgeon is aerating the sinuses and restoring mucociliary clearance in order to restore the function of paranasal sinuses [1].