An Arf6- and Caveolae-Dependent Pathway Links Hemidesmosome Remodeling and Mechanoresponse
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clathrin-Independent Pathways of Endocytosis
Downloaded from http://cshperspectives.cshlp.org/ on October 3, 2021 - Published by Cold Spring Harbor Laboratory Press Clathrin-Independent Pathways of Endocytosis Satyajit Mayor1, Robert G. Parton2, and Julie G. Donaldson3 1National Centre for Biological Sciences, Tata Institute of Fundamental Research, and Institute for Stem Cell Biology and Regenerative Medicine, Bangalore 560065, India 2The University of Queensland, Institute for Molecular Bioscience and Centre for Microscopy and Microanalysis, Queensland 4072, Brisbane, Australia 3Cell Biology and Physiology Center, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland 20892 Correspondence: [email protected] There are many pathways of endocytosis at the cell surface that apparently operate at the same time. With the advent of new molecular genetic and imaging tools, an understanding of the different ways by which a cell may endocytose cargo is increasing by leaps and bounds. In this review we explore pathways of endocytosis that occur in the absence of clathrin. These are referred to as clathrin-independent endocytosis (CIE). Here we primarily focus on those pathways that function at the small scale in which some have distinct coats (caveolae) and others function in the absence of specific coated intermediates. We follow the trafficking itineraries of the material endocytosed by these pathways and finally discuss the functional roles that these pathways play in cell and tissue physiology. It is likely that these pathways will play key roles in the regulation of plasma membrane area and tension and also control the availability of membrane during cell migration. he identification of many of the components Consequently, CME has remained a pre- Tinvolved in clathrin-mediated endocytosis dominant paradigm for following the uptake (CME) and their subsequent characterization of material into the cell. -

Palmitoylation: Implications for Nitric Oxide Signaling
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 6448-6453, June 1996 Cell Biology Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: Implications for nitric oxide signaling (endothelial nitric oxide synthase/signal transduction/vascular biology/N-myristoylation) GUILLERMO GARC1A-CARDENA*, PHIL OHt, JIANwEI LIu*, JAN E. SCHNITZERt, AND WILLIAM C. SESSA*t *Molecular Cardiobiology Program and Department of Pharmacology, Yale University School of Medicine, 295 Congress Avenue, New Haven, CT 06536; and tDepartment of Pathology, Harvard Medical School, Beth Israel Hospital, 330 Brookline Avenue, Boston, MA 02215 Communicated by Vincent T. Marchesi, Yale Univeristy, New Haven, CT, March 13, 1996 (received for review February 5, 1996) ABSTRACT The membrane association of endothelial insoluble membranes (TIM), suggesting that caveolae are nitric oxide synthase (eNOS) plays an important role in the signal processing centers (2-11). Additionally, caveolae have biosynthesis of nitric oxide (NO) in vascular endothelium. been implicated in other important cellular functions, includ- Previously, we have shown that in cultured endothelial cells ing endocytosis, potocytosis, and transcytosis (12, 13). and in intact blood vessels, eNOS is found primarily in the Endothelial nitric oxide synthase (eNOS) is a peripheral perinuclear region of the cells and in discrete regions of the membrane protein that metabolizes L-arginine to nitric oxide plasma membrane, suggesting trafficking of the protein from (NO). NO is a short-lived free radical gas involved in diverse the Golgi to specialized plasma membrane structures. Here, physiological and pathological processes. Endothelial-derived we show that eNOS is found in Triton X-100-insoluble mem- NO is an important paracrine mediator of vascular smooth branes prepared from cultured bovine aortic endothelial cells muscle tone and is an inhibitor of leukocyte adhesion and and colocalizes with caveolin, a coat protein of caveolae, in platelet aggregation (14, 15). -

Endothelial Plasmalemma Vesicle–Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells
Endothelial Plasmalemma Vesicle−Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells This information is current as Raul Elgueta, Dan Tse, Sophie J. Deharvengt, Marcus R. of September 26, 2021. Luciano, Catherine Carriere, Randolph J. Noelle and Radu V. Stan J Immunol 2016; 197:3970-3981; Prepublished online 14 October 2016; doi: 10.4049/jimmunol.1501859 Downloaded from http://www.jimmunol.org/content/197/10/3970 Supplementary http://www.jimmunol.org/content/suppl/2016/10/13/jimmunol.150185 Material 9.DCSupplemental http://www.jimmunol.org/ References This article cites 64 articles, 25 of which you can access for free at: http://www.jimmunol.org/content/197/10/3970.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 26, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Endothelial Plasmalemma Vesicle–Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells Raul Elgueta,*,† Dan Tse,‡,1 Sophie J. -

Membrane Capacitance Recordings Resolve Dynamics and Complexity Of
www.nature.com/scientificreports OPEN Membrane capacitance recordings resolve dynamics and complexity of receptor-mediated endocytosis in Received: 22 October 2018 Accepted: 20 August 2019 Wnt signalling Published: xx xx xxxx Vera Bandmann1, Ann Schirin Mirsanaye1, Johanna Schäfer1, Gerhard Thiel1, Thomas Holstein2 & Melanie Mikosch-Wersching1,2 Receptor-mediated endocytosis is an essential process in signalling pathways for activation of intracellular signalling cascades. One example is the Wnt signalling pathway that seems to depend on endocytosis of the ligand-receptor complex for initiation of Wnt signal transduction. To date, the roles of diferent endocytic pathways in Wnt signalling, molecular players and the kinetics of the process remain unclear. Here, we monitored endocytosis in Wnt3a and Wnt5a-mediated signalling with membrane capacitance recordings of HEK293 cells. Our measurements revealed a swift and substantial increase in the number of endocytic vesicles. Extracellular Wnt ligands specifcally triggered endocytotic activity, which started immediately upon ligand binding and ceased within a period of ten minutes. By using specifc inhibitors, we were able to separate Wnt-induced endocytosis into two independent pathways. We demonstrate that canonical Wnt3a is taken up mainly by clathrin-independent endocytosis whereas noncanonical Wnt5a is exclusively regulated via clathrin-mediated endocytosis. Our fndings show that membrane capacitance recordings allow the resolution of complex cellular processes in plasma membrane signalling pathways in great detail. Wnt signalling is a highly-conserved signalling pathway with important functions in development, tissue-homeostasis, stem cell biology and many diseases, including cancer. Afer three decades of dedicated research we have come to understand many of the fundamental components of Wnt signalling pathways. -
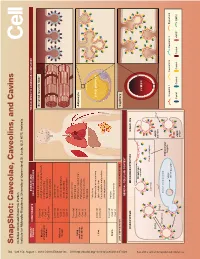
Snapshot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G
704 Cell SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton 154 Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia , August 1,2013©2013 Elsevier Inc. DOI http://dx.doi.org/10.1016/j.cell.2013.07.009 ORGAN/ COMPONENTS DISEASE AND TISSUE-SPECIFIC CAVEOLAR COMPLEXES TISSUE CELLULAR PROCESS Rippling muscle disease Caveolin3 Striated muscle fiber Limb girdle muscular dystrophy Muscle Cavin1 skeletal and Cardiomyopathy Cavin4 cardiac Mechanoprotection Cav1 (heart) T-tubule formation Caveolin1 Lipodystrophy Caveolin2 Lipotoxicity Adipose Cavin1 Fatty acid regulation tissue Cavin2 Insulin signaling Cavin3 Mechanoprotection Atherosclerosis Caveolin1 Inammation Caveolin2 Lung Pulmonary hypertension and other Cavin1 Adipocyte Pulmonary brosis endothelia Cavin2 Mechanosensation Cavin3 Signaling Fatty liver Hepatocellular carcinoma Caveolin1 LIPID DROPLET Liver Lipid metabolism Caveolin2 Carbohydrate metabolism Liver regeneration Caveolin1 Autism Brain Cavin1 Schizophrenia Cavin3 LOW EXPRESSION HIGH EXPRESSION Capillary GENERAL CELLULAR CONTEXT ENDOCYTOSIS MECHANOPROTECTION SIGNALING See online version for legend and references. and legend for version online See Membrane stretch LUMEN Cavin complex Caveola EHD2 eNOS Ca2+ inactive Rab5 Dynamin2 EARLY ENDOSOME Intracellular targets MVB/ Caveolin 1 Caveolin 2 Caveolin 3 Dynamin2 late endosome eNOS Cavin1 Cavin2 Cavin3 Cavin4 eNOS EHD2 active SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia Caveolae, submicroscopic bulb-shaped plasma membrane pits, are an abundant feature of many mammalian cells (Parton and del Pozo, 2013). Caveolae and the major proteins of caveolae, caveolins (Rothberg et al., 1992), and cavins (Hill et al., 2008), are linked to a number of human diseases such as muscular dystrophy, cardiomyopathy, and lipodys- trophy (see Glossary) (Bruno et al., 1993; Fernández et al., 2006; Hayashi et al., 2009). -

Structure and Function of SNARE and SNARE-Interacting Proteins
Quarterly Reviews of Biophysics 38, 1 (2006), pp. 1–47. f 2005 Cambridge University Press 1 doi:10.1017/S0033583505004051 Printed in the United Kingdom First published online 9 December 2005 Structure and functionof SNARE and SNARE-interacting proteins Axel T. Brunger Howard Hughes Medical Institute and Departments of Molecular and Cellular Physiology, Neurology and Neurological Sciences, and Stanford Synchrotron Radiation Laboratory, Stanford University, Stanford, CA 94305, USA Abstract. This review focuses on the so-called SNARE (soluble N-ethyl maleimide sensitive factor attachment protein receptor) proteins that are involved in exocytosis at the pre-synpatic plasma membrane. SNAREs play a role in docking and fusion of synaptic vesicles to the active zone, as well as in the Ca2+-triggering step itself, most likely in combination with the Ca2+ sensor synaptotagmin. Different SNARE domains are involved in different processes, such as regulation, docking, and fusion. SNAREs exhibit multiple configurational, conformational, and oliogomeric states. These different states allow SNAREs to interact with their matching SNARE partners, auxiliary proteins, or with other SNARE domains, often in a mutually exclusive fashion. SNARE core domains undergo progressive disorder to order transitions upon interactions with other proteins, culminating with the fully folded post-fusion (cis) SNARE complex. Physiological concentrations of neuronal SNAREs can juxtapose membranes, and promote fusion in vitro under certain conditions. However, significantly more -
Modulation of the Caveolin-3 Localization to Caveolae and STAT3 to Mitochondria by Catecholamine-Induced Cardiac Hypertrophy in H9c2 Cardiomyoblasts
EXPERIMENTAL and MOLECULAR MEDICINE, Vol. 41, No. 4, 226-235, April 2009 Modulation of the caveolin-3 localization to caveolae and STAT3 to mitochondria by catecholamine-induced cardiac hypertrophy in H9c2 cardiomyoblasts Kyuho Jeong*, Hayeong Kwon*, amine-induced cardiac hypertrophy. Chanhee Min and Yunbae Pak1 Keywords: cardiomegaly; caveolae; caveolin-3; cell Department of Biochemistry nucleus; heart; isoproterenol; mitochondria; phenyl- Division of Applied Life Science (BK21), PMBBRC ephrine; STAT3 transcription factor Environmental Biotechnology National Core Research Center Gyeongsang National University Jinju 660-701, Korea Introduction 1Corresponding author: Tel, 82-55-751-5961; Fax, 82-55-759-9363; E-mail, [email protected] Hypertension is major risk factors for cardiac da- mage, ischemia, myocardial infarction, and conge- *These authors contributed equally to this work. stive heart failure (Zampaglione et al., 1996). In DOI 10.3858/emm.2009.41.4.025 response to increased demands for cardiac work caused by various pathologic stresses, heart adapts Accepted 20 November 2008 through compensatory hypertrophy of myocytes. Abbreviations: Akt, protein kinase B; CsA, cyclosporin A; GPCR, Thus, cardiac hypertrophy is recognized in many G protein-coupled receptor; ISO, isoproterenol; PE, phenylephrine; cardiovascular diseases, such as hypertension, RTK, receptor tyrosine kinase; STAT3, signal transducers and acti- vascular disease, and myocardial infarction, and is vator of transcription 3 an independent risk factor for cardiac morbidity and mortality. Hypertrophic stimuli induce an in- crease in cell size in the absence of cell division through Ca2+ signaling and activation of PKC, Abstract MAPK and PKB/ Akt (Watanabe et al., 2001; Dorn and Force, 2005), and are accompanied by We investigated the effect of phenylephrine (PE)- and increased protein synthesis with reprogramming of isoproterenol (ISO)-induced cardiac hypertrophy on gene expression (Takeo et al., 2000). -

Three Ways to Make a Vesicle
REVIEWS THREE WAYS TO MAKE A VESICLE Tomas Kirchhausen Cargo molecules have to be included in carrier vesicles of different forms and sizes to be transported between organelles. During this process, a limited set of proteins, including the coat proteins COPI, COPII and clathrin, carries out a programmed set of sequential interactions that lead to the budding of vesicles. A general model to explain the formation of coated vesicles is starting to emerge but the picture is more complex than we had imagined. ENDOCYTIC PATHWAY Transport of proteins and lipids along the ENDOCYTIC or energy dependent and includes sorting of cargo to the Macromolecules are taken up SECRETORY PATHWAYS is a hallmark of eukaryotic cells. The forming coat. Coat propagation, the second step in the by invagination of the plasma membrane fluxes along these pathways are very large process, couples further addition of coat components membrane. They first arrive in and rapid. A FIBROBLAST kept in resting conditions in a and additional recruitment of cargo with invagination of early endosomes, then late endosomes, and finally tissue culture plate internalizes an amount of mem- the underlying membrane. When formation of the coat lysosomes, where they are brane equivalent to the whole surface area of the cell in ends, the vesicle buds by scission of the neck connecting degraded by hydrolases. one hour. Inside the cell, it often takes only seconds for a the deeply invaginated membrane to the donor surface. carrier vesicle to move from the donor membrane to This is a relatively simple step for COPI- and COPII- SECRETORY PATHWAY the acceptor organelle. -

A Clathrin Coat Assembly Role for the Muniscin Protein Central Linker
1 A clathrin coat assembly role for the muniscin protein central linker 2 revealed by TALEN-mediated gene editing 3 4 Perunthottathu K. Umasankar1, Li Ma1,4, James R. Thieman1,,5, Anupma Jha1, 5 Balraj Doray2, Simon C. Watkins1 and Linton M. Traub1,3 6 7 From the 1Department of Cell Biology 8 University of Pittsburgh School of Medicine 9 10 2Department of Medicine 11 Washington University School of Medicine 12 13 3 To whom correspondence should be addressed at: 14 Department of Cell Biology 15 University of Pittsburgh School of Medicine 16 3500 Terrace Street, S3012 BST 17 Pittsburgh, PA 15261 18 Tel: (412) 648-9711 19 FaX: (412) 648-9095 20 e-mail: [email protected] 21 22 4 Current address: 23 Tsinghua University School of Medicine 24 Beijing, China 25 26 5 Current address 27 Olympus America, Inc. 28 Center Valley, PA 18034 29 1 29 Abstract 30 31 Clathrin-mediated endocytosis is an evolutionarily ancient membrane transport system 32 regulating cellular receptivity and responsiveness. Plasmalemma clathrin-coated 33 structures range from unitary domed assemblies to eXpansive planar constructions with 34 internal or flanking invaginated buds. Precisely how these morphologically-distinct coats 35 are formed, and whether all are functionally equivalent for selective cargo internalization is 36 still disputed. We have disrupted the genes encoding a set of early arriving clathrin-coat 37 constituents, FCHO1 and FCHO2, in HeLa cells. Endocytic coats do not disappear in this 38 genetic background; rather clustered planar lattices predominate and endocytosis slows, 39 but does not cease. The central linker of FCHO proteins acts as an allosteric regulator of the 40 prime endocytic adaptor, AP-2. -

The Life Cycle of a Transport Vesicle
Cell. Mol. Life Sci. 65 (2008) 2781 – 2789 View metadata,1420-682X/08/182781-9 citation and similar papers at core.ac.uk Cellular and Molecular Life Sciencesbrought to you by CORE DOI 10.1007/s00018-008-8349-y provided by RERO DOC Digital Library Birkhuser Verlag, Basel, 2008 The life cycle of a transport vesicle A. Spang Biozentrum, Growth and Development, University of Basel, Klingelbergstrasse 70, 4056 Basel (Switzerland), e-mail: [email protected], Fax: +41 61 267 2148 Online First 26 August 2008 Abstract. Vesicular transport is the basic communica- These additional coats may generate vesicles that tion mechanism between different compartments in a transport cargo in a temporally and/or spatially cell and with the environment. In this review I discuss controlled manner. Work over the last years suggests the principles of vesicle generation and consumption that GTP hydrolysis occurs early during vesicle bio- with particular emphasis on the different types of coat genesis, destabilizing the coat perhaps before fission proteins and the timing of the shedding of the coat of the vesicle from the donor membrane occurs. proteins from transport containers. In recent years it Recent findings imply, however, that tethers at the has become clear that there are more coat complexes receiving compartment specifically detect the coat on than the classical COPI, COPII and clathrin coats. vesicle. (Part of a Multi-author Review) Keywords. Vesicle biogenesis, small GTPases, coat proteins, SNAREs, Arf1, coatomer, adaptor complex, clathrin, COPII, COPI, Sar1. Why is vesicular transport important? impair the function of the encoded protein, because a complete loss of function would lead to embryonic Communication between both diverse membrane- lethality. -

Endocytosis: the Nanoparticle and Submicron Nanocompounds Gateway Into the Cell
pharmaceutics Review Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell Darío Manzanares 1,2 and Valentín Ceña 1,2,* 1 Unidad Asociada Neurodeath, Universidad de Castilla-La Mancha, 02006 Albacete, Spain; [email protected] 2 CIBERNED, Instituto de Salud Carlos III, 28031 Madrid, Spain * Correspondence: [email protected] Received: 23 March 2020; Accepted: 15 April 2020; Published: 17 April 2020 Abstract: Nanoparticles (NPs) and submicron particles are increasingly used as carriers for delivering therapeutic compounds to cells. Their entry into the cell represents the initial step in this delivery process, being most of the nanoparticles taken up by endocytosis, although other mechanisms can contribute to the uptake. To increase the delivery efficiency of therapeutic compounds by NPs and submicron particles is very relevant to understand the mechanisms involved in the uptake process. This review covers the proposed pathways involved in the cellular uptake of different NPs and submicron particles types as well as the role that some of the physicochemical nanoparticle characteristics play in the uptake pathway preferentially used by the nanoparticles to gain access and deliver their cargo inside the cell. Keywords: nanoparticles; endocytosis; clathrin; caveolin; macropinocytosis 1. Introduction Nanomedicine has become one of the most rapidly growing areas of research in the biomedical field during the last years. Nanoparticles (NPs) and submicron particles (named both from this point as NPs to abbreviate) are defined as materials with nanometric sizes (1–100 and 100–1000 nm, respectively) that interact with biological systems in an unusual way because of their high surface to volume ratio. This property, combined with the possibility of modifying their peripheral chemical groups to achieve multitasking properties, provides NPs compounds with a very high potential for diagnostic and therapeutic applications in nanomedicine. -

Kinetic Regulation of Coated Vesicle Secretion
Kinetic regulation of coated vesicle secretion Lionel Foret ∗ and Pierre Sens † ∗Max-Planck-Institute for the Physics of Complex Systems, Nothnitzer Strasse 38, 01187 Dresden, Germany, and †ESPCI, rue Vaucquelin, 75005 Paris Submitted to Proceedings of the National Academy of Sciences of the United States of America The secretion of vesicles for intracellular transport often rely on taneous, only driven by weak short range attractions between the the aggregation of specialized membrane-bound proteins into a monomers, while coat disassembly requires the presence of an en- coat able to curve cell membranes. The nucleation and growth of ergy source. More precisely, the assembly and disassembly of COP a protein coat is a kinetic process that competes with the energy- consuming turnover of coat components between the membrane coat components follow the cycle of activation - inactivation of a GT- and the cytosol. We propose a generic kinetic description of coat Pase protein, Sar1 for COPII and Arf1 for COPI [4]. Once activated, assembly and the formation of coated vesicles, and discuss its the GTPases bind to the membrane and recruit individual coatomer implication to the dynamics of COP vesicles that traffic within the complexes (the monomers), that later polymerize into coats [10]. The Golgi and with the Endoplasmic Reticulum. We show that station- inactivation of the GTPase, triggered by the hydrolysis of its bound ary coats of fixed area emerge from the competition between coat growth and the recycling of coat components, in a fashion resem- GTP, leads to its unbinding from the membrane and to the monomer bling the treadmilling of cytoskeletal filaments.