Tissue Distribution of Caveolin 1399
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clathrin-Independent Pathways of Endocytosis
Downloaded from http://cshperspectives.cshlp.org/ on October 3, 2021 - Published by Cold Spring Harbor Laboratory Press Clathrin-Independent Pathways of Endocytosis Satyajit Mayor1, Robert G. Parton2, and Julie G. Donaldson3 1National Centre for Biological Sciences, Tata Institute of Fundamental Research, and Institute for Stem Cell Biology and Regenerative Medicine, Bangalore 560065, India 2The University of Queensland, Institute for Molecular Bioscience and Centre for Microscopy and Microanalysis, Queensland 4072, Brisbane, Australia 3Cell Biology and Physiology Center, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland 20892 Correspondence: [email protected] There are many pathways of endocytosis at the cell surface that apparently operate at the same time. With the advent of new molecular genetic and imaging tools, an understanding of the different ways by which a cell may endocytose cargo is increasing by leaps and bounds. In this review we explore pathways of endocytosis that occur in the absence of clathrin. These are referred to as clathrin-independent endocytosis (CIE). Here we primarily focus on those pathways that function at the small scale in which some have distinct coats (caveolae) and others function in the absence of specific coated intermediates. We follow the trafficking itineraries of the material endocytosed by these pathways and finally discuss the functional roles that these pathways play in cell and tissue physiology. It is likely that these pathways will play key roles in the regulation of plasma membrane area and tension and also control the availability of membrane during cell migration. he identification of many of the components Consequently, CME has remained a pre- Tinvolved in clathrin-mediated endocytosis dominant paradigm for following the uptake (CME) and their subsequent characterization of material into the cell. -

Functions of Vertebrate Ferlins
cells Review Functions of Vertebrate Ferlins Anna V. Bulankina 1 and Sven Thoms 2,* 1 Department of Internal Medicine 1, Goethe University Hospital Frankfurt, 60590 Frankfurt, Germany; [email protected] 2 Department of Child and Adolescent Health, University Medical Center Göttingen, 37075 Göttingen, Germany * Correspondence: [email protected] Received: 27 January 2020; Accepted: 20 February 2020; Published: 25 February 2020 Abstract: Ferlins are multiple-C2-domain proteins involved in Ca2+-triggered membrane dynamics within the secretory, endocytic and lysosomal pathways. In bony vertebrates there are six ferlin genes encoding, in humans, dysferlin, otoferlin, myoferlin, Fer1L5 and 6 and the long noncoding RNA Fer1L4. Mutations in DYSF (dysferlin) can cause a range of muscle diseases with various clinical manifestations collectively known as dysferlinopathies, including limb-girdle muscular dystrophy type 2B (LGMD2B) and Miyoshi myopathy. A mutation in MYOF (myoferlin) was linked to a muscular dystrophy accompanied by cardiomyopathy. Mutations in OTOF (otoferlin) can be the cause of nonsyndromic deafness DFNB9. Dysregulated expression of any human ferlin may be associated with development of cancer. This review provides a detailed description of functions of the vertebrate ferlins with a focus on muscle ferlins and discusses the mechanisms leading to disease development. Keywords: dysferlin; myoferlin; otoferlin; C2 domain; calcium-sensor; muscular dystrophy; dysferlinopathy; limb girdle muscular dystrophy type 2B (LGMD2B), membrane repair; T-tubule system; DFNB9 1. Introduction Ferlins belong to the superfamily of proteins with multiple C2 domains (MC2D) that share common functions in tethering membrane-bound organelles or recruiting proteins to cellular membranes. Ferlins are described as calcium ions (Ca2+)-sensors for vesicular trafficking capable of sculpturing membranes [1–3]. -

Caveolin-1 Is Down-Regulated in Alveolar Rhabdomyosarcomas and Negatively Regulates Tumor Growth
www.impactjournals.com/oncotarget/ Oncotarget, Vol. 5, No. 20 Caveolin-1 is down-regulated in alveolar rhabdomyosarcomas and negatively regulates tumor growth Juan Huertas-Martínez1, Santiago Rello-Varona1, David Herrero-Martín1, Ignasi Barrau1, Silvia García-Monclús1, Miguel Sáinz-Jaspeado1, Laura Lagares-Tena1, Yaiza Núñez-Álvarez5, Silvia Mateo-Lozano2, Jaume Mora2, Josep Roma3, Nuria Toran3, Sebastian Moran4, Roser López-Alemany1, Soledad Gallego3, Manel Esteller4, Miguel A. Peinado5, Xavier García del Muro1 and Oscar M. Tirado1 1 Sarcoma research group, Molecular Oncology Lab, Bellvitge Biomedical Research Institute (IDIBELL), L’Hospitalet de Llobregat, Barcelona, Spain 2 Developmental Tumor Biology Laboratory, Hospital Sant Joan de Deu, Barcelona, Spain 3 Biomedical Research Unit, Hospital Universitari Vall d’Hebron, Barcelona, Spain 4 Cancer Epigenetics and Biology Programme (PEBC), Bellvitge Biomedical Research Institute (IDIBELL), L’ Hospitalet de Llobregat, Barcelona, Spain 5 Institut de Medicina Predictiva i Personalitzada del Càncer, Badalona, Barcelona, Spain Correspondence to: Oscar M. Tirado, email: [email protected] Keywords: alveolar rhabdomyosarcoma, Caveolin-1, muscular differentiation, 5-AZA-2’-deoxycytidine, epigenetics, cell death Received: June 26, 2014 Accepted: August 26, 2014 Published: August 27, 2014 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT Rhabdomyosarcoma is the most common soft tissue sarcoma of childhood and adolescence. Despite advances in therapy, patients with histological variant of rhabdomyosarcoma known as alveolar rhabdomyosarcoma (ARMS) have a 5-year survival of less than 30%. Caveolin-1 (CAV1), encoding the structural component of cellular caveolae, is a suggested tumor suppressor gene involved in cell signaling. -

Palmitoylation: Implications for Nitric Oxide Signaling
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 6448-6453, June 1996 Cell Biology Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: Implications for nitric oxide signaling (endothelial nitric oxide synthase/signal transduction/vascular biology/N-myristoylation) GUILLERMO GARC1A-CARDENA*, PHIL OHt, JIANwEI LIu*, JAN E. SCHNITZERt, AND WILLIAM C. SESSA*t *Molecular Cardiobiology Program and Department of Pharmacology, Yale University School of Medicine, 295 Congress Avenue, New Haven, CT 06536; and tDepartment of Pathology, Harvard Medical School, Beth Israel Hospital, 330 Brookline Avenue, Boston, MA 02215 Communicated by Vincent T. Marchesi, Yale Univeristy, New Haven, CT, March 13, 1996 (received for review February 5, 1996) ABSTRACT The membrane association of endothelial insoluble membranes (TIM), suggesting that caveolae are nitric oxide synthase (eNOS) plays an important role in the signal processing centers (2-11). Additionally, caveolae have biosynthesis of nitric oxide (NO) in vascular endothelium. been implicated in other important cellular functions, includ- Previously, we have shown that in cultured endothelial cells ing endocytosis, potocytosis, and transcytosis (12, 13). and in intact blood vessels, eNOS is found primarily in the Endothelial nitric oxide synthase (eNOS) is a peripheral perinuclear region of the cells and in discrete regions of the membrane protein that metabolizes L-arginine to nitric oxide plasma membrane, suggesting trafficking of the protein from (NO). NO is a short-lived free radical gas involved in diverse the Golgi to specialized plasma membrane structures. Here, physiological and pathological processes. Endothelial-derived we show that eNOS is found in Triton X-100-insoluble mem- NO is an important paracrine mediator of vascular smooth branes prepared from cultured bovine aortic endothelial cells muscle tone and is an inhibitor of leukocyte adhesion and and colocalizes with caveolin, a coat protein of caveolae, in platelet aggregation (14, 15). -

Endothelial Plasmalemma Vesicle–Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells
Endothelial Plasmalemma Vesicle−Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells This information is current as Raul Elgueta, Dan Tse, Sophie J. Deharvengt, Marcus R. of September 26, 2021. Luciano, Catherine Carriere, Randolph J. Noelle and Radu V. Stan J Immunol 2016; 197:3970-3981; Prepublished online 14 October 2016; doi: 10.4049/jimmunol.1501859 Downloaded from http://www.jimmunol.org/content/197/10/3970 Supplementary http://www.jimmunol.org/content/suppl/2016/10/13/jimmunol.150185 Material 9.DCSupplemental http://www.jimmunol.org/ References This article cites 64 articles, 25 of which you can access for free at: http://www.jimmunol.org/content/197/10/3970.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 26, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Endothelial Plasmalemma Vesicle–Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells Raul Elgueta,*,† Dan Tse,‡,1 Sophie J. -

Novel Missense Mutation in the Caveolin-3 Gene in a Belgian Family
1349 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2003.028217 on 16 August 2004. Downloaded from SHORT REPORT Novel missense mutation in the caveolin-3 gene in a Belgian family with rippling muscle disease P Y K Van den Bergh, J M Ge´rard, J A Elosegi, M U Manto, C Kubisch, B G H Schoser ............................................................................................................................... J Neurol Neurosurg Psychiatry 2004;75:1349–1351. doi: 10.1136/jnnp.2003.028217 too small’’). The patient was diagnosed as having fibromyal- Rippling muscle disease (RMD) is a rare muscle disorder gia, which led to secondary depression and loss of her job. characterised by muscle stiffness, exercise induced myalgia, Muscle weakness and pigmenturia were absent. The medical and cramp-like sensations. It is genetically heterogeneous history was remarkable for mild hypothyroidism, peptic and can be acquired, but most cases show autosomal oesophagitis, and tobacco related asthmatiform bronchitis dominant inheritance due to mutations in the caveolin-3 with emphysema. At age 38, she had a neurological work-up. (CAV3) gene. We report a novel heterozygous missense The cranial nerves, muscle bulk, muscle strength, and deep mutation in CAV3 in a Belgian family with autosomal tendon reflexes were normal, and plantar responses were dominant RMD. flexor. Tapping with the reflex hammer or with the finger on A 40 year old woman complained of fatigue, exercise the muscles provoked an immediate, short lasting, forceful induced muscle pain, and muscle cramps since the age of 35. contraction and/or painful local mounding lasting for several Neurological examination revealed percussion induced seconds. PIRCs were most pronounced in the sternocleido- rapid muscle contractions (PIRCs) and localised muscle mastoid, deltoid, biceps, brachioradialis, finger and wrist mounding on percussion; muscle rippling was not observed. -

The Popeye Domain Containing Genes and Their Function in Striated Muscle
Journal of Cardiovascular Development and Disease Review The Popeye Domain Containing Genes and Their Function in Striated Muscle Roland F. R. Schindler 1, Chiara Scotton 2, Vanessa French 1, Alessandra Ferlini 2 and Thomas Brand 1,* 1 Developmental Dynamics, Harefield Heart Science Centre, National Heart and Lung Institute, Imperial College London, Hill End Road, Harefield UB9 6JH, UK; [email protected] (R.F.R.S.); [email protected] (V.F.) 2 Department of Medical Sciences, Medical Genetics Unit, University of Ferrara, Ferrara 44121, Italy; [email protected] (C.S.); fl[email protected] (A.F.) * Correspondence: [email protected]; Tel.: +44-189-582-8900 Academic Editors: Robert E. Poelmann and Monique R.M. Jongbloed Received: 26 April 2016; Accepted: 13 June 2016; Published: 15 June 2016 Abstract: The Popeye domain containing (POPDC) genes encode a novel class of cAMP effector proteins, which are abundantly expressed in heart and skeletal muscle. Here, we will review their role in striated muscle as deduced from work in cell and animal models and the recent analysis of patients carrying a missense mutation in POPDC1. Evidence suggests that POPDC proteins control membrane trafficking of interacting proteins. Furthermore, we will discuss the current catalogue of established protein-protein interactions. In recent years, the number of POPDC-interacting proteins has been rising and currently includes ion channels (TREK-1), sarcolemma-associated proteins serving functions in mechanical stability (dystrophin), compartmentalization (caveolin 3), scaffolding (ZO-1), trafficking (NDRG4, VAMP2/3) and repair (dysferlin) or acting as a guanine nucleotide exchange factor for Rho-family GTPases (GEFT). -

Membrane Capacitance Recordings Resolve Dynamics and Complexity Of
www.nature.com/scientificreports OPEN Membrane capacitance recordings resolve dynamics and complexity of receptor-mediated endocytosis in Received: 22 October 2018 Accepted: 20 August 2019 Wnt signalling Published: xx xx xxxx Vera Bandmann1, Ann Schirin Mirsanaye1, Johanna Schäfer1, Gerhard Thiel1, Thomas Holstein2 & Melanie Mikosch-Wersching1,2 Receptor-mediated endocytosis is an essential process in signalling pathways for activation of intracellular signalling cascades. One example is the Wnt signalling pathway that seems to depend on endocytosis of the ligand-receptor complex for initiation of Wnt signal transduction. To date, the roles of diferent endocytic pathways in Wnt signalling, molecular players and the kinetics of the process remain unclear. Here, we monitored endocytosis in Wnt3a and Wnt5a-mediated signalling with membrane capacitance recordings of HEK293 cells. Our measurements revealed a swift and substantial increase in the number of endocytic vesicles. Extracellular Wnt ligands specifcally triggered endocytotic activity, which started immediately upon ligand binding and ceased within a period of ten minutes. By using specifc inhibitors, we were able to separate Wnt-induced endocytosis into two independent pathways. We demonstrate that canonical Wnt3a is taken up mainly by clathrin-independent endocytosis whereas noncanonical Wnt5a is exclusively regulated via clathrin-mediated endocytosis. Our fndings show that membrane capacitance recordings allow the resolution of complex cellular processes in plasma membrane signalling pathways in great detail. Wnt signalling is a highly-conserved signalling pathway with important functions in development, tissue-homeostasis, stem cell biology and many diseases, including cancer. Afer three decades of dedicated research we have come to understand many of the fundamental components of Wnt signalling pathways. -
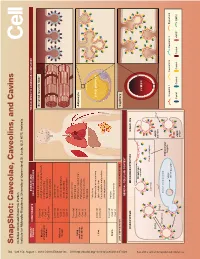
Snapshot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G
704 Cell SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton 154 Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia , August 1,2013©2013 Elsevier Inc. DOI http://dx.doi.org/10.1016/j.cell.2013.07.009 ORGAN/ COMPONENTS DISEASE AND TISSUE-SPECIFIC CAVEOLAR COMPLEXES TISSUE CELLULAR PROCESS Rippling muscle disease Caveolin3 Striated muscle fiber Limb girdle muscular dystrophy Muscle Cavin1 skeletal and Cardiomyopathy Cavin4 cardiac Mechanoprotection Cav1 (heart) T-tubule formation Caveolin1 Lipodystrophy Caveolin2 Lipotoxicity Adipose Cavin1 Fatty acid regulation tissue Cavin2 Insulin signaling Cavin3 Mechanoprotection Atherosclerosis Caveolin1 Inammation Caveolin2 Lung Pulmonary hypertension and other Cavin1 Adipocyte Pulmonary brosis endothelia Cavin2 Mechanosensation Cavin3 Signaling Fatty liver Hepatocellular carcinoma Caveolin1 LIPID DROPLET Liver Lipid metabolism Caveolin2 Carbohydrate metabolism Liver regeneration Caveolin1 Autism Brain Cavin1 Schizophrenia Cavin3 LOW EXPRESSION HIGH EXPRESSION Capillary GENERAL CELLULAR CONTEXT ENDOCYTOSIS MECHANOPROTECTION SIGNALING See online version for legend and references. and legend for version online See Membrane stretch LUMEN Cavin complex Caveola EHD2 eNOS Ca2+ inactive Rab5 Dynamin2 EARLY ENDOSOME Intracellular targets MVB/ Caveolin 1 Caveolin 2 Caveolin 3 Dynamin2 late endosome eNOS Cavin1 Cavin2 Cavin3 Cavin4 eNOS EHD2 active SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia Caveolae, submicroscopic bulb-shaped plasma membrane pits, are an abundant feature of many mammalian cells (Parton and del Pozo, 2013). Caveolae and the major proteins of caveolae, caveolins (Rothberg et al., 1992), and cavins (Hill et al., 2008), are linked to a number of human diseases such as muscular dystrophy, cardiomyopathy, and lipodys- trophy (see Glossary) (Bruno et al., 1993; Fernández et al., 2006; Hayashi et al., 2009). -

Development of an Infectious Clone System to Study the Life Cycle of Hazara Virus
Development of an Infectious Clone System to Study the Life Cycle of Hazara Virus Jack Fuller Submitted in accordance with the requirements for the degree of Doctor of Philosophy The University of Leeds Faculty of Biological Sciences July 2020 i The candidate confirms that the work submitted is his own and that appropriate credit has been given where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. © 2020 The University of Leeds and Jack Fuller ii Acknowledgements I would like to thank my supervisors, John, Jamel and Roger for their endless support and fresh ideas throughout my PhD. A special thanks go to John for always providing enthusiasm and encouragement, especially during the tougher times of the project! I would also like to thank everyone in 8.61, not only for making my time at Leeds enjoyable, but for helping me develop as a scientist through useful critique and discussion. A special thanks goes to Francis Hopkins, for welcoming me into the Barr group and providing a constant supply of humour and to Ellie Todd, for listening to my endless whines and gripes, and for providing welcome distractions in the form of her PowerPoint artwork. Outside of the lab, my mum deserves a special mention for always being the voice of reason and support at the end of the phone, but also for pushing me to succeed throughout my entire education. I have no doubts I would not be in the position I am now without her! Finally, a huge thanks to my partner Hannah, who has been there to support me through all the highs and lows of my PhD, and has sacrificed many weekend trips to allow me to finish experiments and to tend to my viruses! iii Abstract Crimean-Congo hemorrhagic fever orthonairovirus (CCHFV) is a negative sense single stranded RNA virus, capable of causing fatal hemorrhagic fever in humans. -

Caveolar Endocytosis of Simian Virus 40 Reveals a New Two-Step Vesicular- Transport Pathway to the ER
articles Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular- transport pathway to the ER Lucas Pelkmans*, Jürgen Kartenbeck† and Ari Helenius*‡ *Institute of Biochemistry, Swiss Federal Institute of Technology, Universitaetstrasse 16, CH-8092 Zürich, Switzerland †German Cancer Research Center (DKFZ) Heidelberg, Im Neuenheimer Feld 280, D-69120 Heidelberg, Germany ‡e-mail: [email protected] Simian virus 40 (SV40) is unusual among animal viruses in that it enters cells through caveolae, and the internalized virus accumulates in a smooth endoplasmic reticulum (ER) compartment. Using video-enhanced, dual-colour, live fluorescence microscopy, we show the uptake of individual virus particles in CV-1 cells. After associating with cave- olae, SV40 leaves the plasma membrane in small, caveolin-1-containing vesicles. It then enters larger, peripheral organelles with a non-acidic pH. Although rich in caveolin-1, these organelles do not contain markers for endo- somes, lysosomes, ER or Golgi, nor do they acquire ligands of clathrin-coated vesicle endocytosis. After several hours in these organelles, SV40 is sorted into tubular, caveolin-free membrane vesicles that move rapidly along microtubules, and is deposited in perinuclear, syntaxin 17-positive, smooth ER organelles. The microtubule-disrupt- ing agent nocodazole inhibits formation and transport of these tubular carriers, and blocks viral infection. Our results demonstrate the existence of a two-step transport pathway from plasma-membrane caveolae, through an intermediate organelle (termed the caveosome), to the ER. This pathway bypasses endosomes and the Golgi com- plex, and is part of the productive infectious route used by SV40. any animal viruses take advantage of receptor-mediated mutants of caveolin-3 localize to intracellular vesicles that are dis- endocytosis to enter their host cells. -

Structure and Function of SNARE and SNARE-Interacting Proteins
Quarterly Reviews of Biophysics 38, 1 (2006), pp. 1–47. f 2005 Cambridge University Press 1 doi:10.1017/S0033583505004051 Printed in the United Kingdom First published online 9 December 2005 Structure and functionof SNARE and SNARE-interacting proteins Axel T. Brunger Howard Hughes Medical Institute and Departments of Molecular and Cellular Physiology, Neurology and Neurological Sciences, and Stanford Synchrotron Radiation Laboratory, Stanford University, Stanford, CA 94305, USA Abstract. This review focuses on the so-called SNARE (soluble N-ethyl maleimide sensitive factor attachment protein receptor) proteins that are involved in exocytosis at the pre-synpatic plasma membrane. SNAREs play a role in docking and fusion of synaptic vesicles to the active zone, as well as in the Ca2+-triggering step itself, most likely in combination with the Ca2+ sensor synaptotagmin. Different SNARE domains are involved in different processes, such as regulation, docking, and fusion. SNAREs exhibit multiple configurational, conformational, and oliogomeric states. These different states allow SNAREs to interact with their matching SNARE partners, auxiliary proteins, or with other SNARE domains, often in a mutually exclusive fashion. SNARE core domains undergo progressive disorder to order transitions upon interactions with other proteins, culminating with the fully folded post-fusion (cis) SNARE complex. Physiological concentrations of neuronal SNAREs can juxtapose membranes, and promote fusion in vitro under certain conditions. However, significantly more