Immunolocalization of Caveolin-1 and Caveolin-3 in Monkey Skeletal, Cardiac and Uterine Smooth Muscles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abl Family Tyrosine Kinases Govern Igg Extravasation in the Skin in a Murine Pemphigus Model
ARTICLE https://doi.org/10.1038/s41467-019-12232-3 OPEN Abl family tyrosine kinases govern IgG extravasation in the skin in a murine pemphigus model Sachiko Ono1, Gyohei Egawa1, Takashi Nomura1, Akihiko Kitoh1, Teruki Dainichi 1, Atsushi Otsuka1, Saeko Nakajima1, Masayuki Amagai2, Fumi Matsumoto3, Mami Yamamoto 3, Yoshiaki Kubota4, Toshiyuki Takai5, Tetsuya Honda1 & Kenji Kabashima 1,6 1234567890():,; The pathway of homeostatic IgG extravasation is not fully understood, in spite of its importance for the maintenance of host immunity, the management of autoantibody- mediated disorders, and the use of antibody-based biologics. Here we show in a murine model of pemphigus, a prototypic cutaneous autoantibody-mediated disorder, that blood- circulating IgG extravasates into the skin in a time- and dose-dependent manner under homeostatic conditions. This IgG extravasation is unaffected by depletion of Fcγ receptors, but is largely attenuated by specific ablation of dynamin-dependent endocytic vesicle for- mation in blood endothelial cells (BECs). Among dynamin-dependent endocytic vesicles, IgG co-localizes well with caveolae in cultured BECs. An Abl family tyrosine kinase inhibitor imatinib, which reduces caveolae-mediated endocytosis, impairs IgG extravasation in the skin and attenuates the murine pemphigus manifestations. Our study highlights the kinetics of IgG extravasation in vivo, which might be a clue to understand the pathological mechanism of autoantibody-mediated autoimmune disorders. 1 Department of Dermatology, Kyoto University Graduate School of Medicine, Kyoto, Japan. 2 Department of Dermatology, Keio University Graduate School of Medicine, Tokyo, Japan. 3 Research Unit/Immunology & Inflammation, Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, Yokohama, Japan. 4 Department of Anatomy, Keio University School of Medicine, Tokyo, Japan. -

Functions of Vertebrate Ferlins
cells Review Functions of Vertebrate Ferlins Anna V. Bulankina 1 and Sven Thoms 2,* 1 Department of Internal Medicine 1, Goethe University Hospital Frankfurt, 60590 Frankfurt, Germany; [email protected] 2 Department of Child and Adolescent Health, University Medical Center Göttingen, 37075 Göttingen, Germany * Correspondence: [email protected] Received: 27 January 2020; Accepted: 20 February 2020; Published: 25 February 2020 Abstract: Ferlins are multiple-C2-domain proteins involved in Ca2+-triggered membrane dynamics within the secretory, endocytic and lysosomal pathways. In bony vertebrates there are six ferlin genes encoding, in humans, dysferlin, otoferlin, myoferlin, Fer1L5 and 6 and the long noncoding RNA Fer1L4. Mutations in DYSF (dysferlin) can cause a range of muscle diseases with various clinical manifestations collectively known as dysferlinopathies, including limb-girdle muscular dystrophy type 2B (LGMD2B) and Miyoshi myopathy. A mutation in MYOF (myoferlin) was linked to a muscular dystrophy accompanied by cardiomyopathy. Mutations in OTOF (otoferlin) can be the cause of nonsyndromic deafness DFNB9. Dysregulated expression of any human ferlin may be associated with development of cancer. This review provides a detailed description of functions of the vertebrate ferlins with a focus on muscle ferlins and discusses the mechanisms leading to disease development. Keywords: dysferlin; myoferlin; otoferlin; C2 domain; calcium-sensor; muscular dystrophy; dysferlinopathy; limb girdle muscular dystrophy type 2B (LGMD2B), membrane repair; T-tubule system; DFNB9 1. Introduction Ferlins belong to the superfamily of proteins with multiple C2 domains (MC2D) that share common functions in tethering membrane-bound organelles or recruiting proteins to cellular membranes. Ferlins are described as calcium ions (Ca2+)-sensors for vesicular trafficking capable of sculpturing membranes [1–3]. -

The Mir-199–Dynamin Regulatory Axis Controls Receptor-Mediated Endocytosis Juan F
© 2015. Published by The Company of Biologists Ltd | Journal of Cell Science (2015) 128, 3197-3209 doi:10.1242/jcs.165233 RESEARCH ARTICLE The miR-199–dynamin regulatory axis controls receptor-mediated endocytosis Juan F. Aranda1,2, Alberto Canfrán-Duque1,2, Leigh Goedeke1,2, Yajaira Suárez1,2 and Carlos Fernández-Hernando1,2,* ABSTRACT mechanism for the selective uptake of essential nutrients such as Small non-coding RNAs (microRNAs) are important regulators of low-density lipoprotein (LDL), through the LDL receptor (LDLR) gene expression that modulate many physiological processes; (Brown and Goldstein, 1986), or iron, through transferrin receptor however, their role in regulating intracellular transport remains (TfR) (Harding et al., 1983). Thus, factors that affect RME have a largely unknown. Intriguingly, we found that the dynamin (DNM) direct effect on these receptors, and, in the case of LDLR, to regulate genes, a GTPase family of proteins responsible for endocytosis in intracellular cholesterol levels. In both the LDLR and TfR eukaryotic cells, encode the conserved miR-199a and miR-199b internalization processes, clathrin plays a key role during the family of miRNAs within their intronic sequences. Here, we formation of coated vesicles (Moore et al., 1987). Once vesicles are demonstrate that miR-199a and miR-199b regulate endocytic internalized, their passage through a broad endosomal compartment transport by controlling the expression of important mediators of system is required; first they are rapidly transported into early endocytosis such as clathrin heavy chain (CLTC), Rab5A, low- endosomes, where Rab5A is a key regulator (Nielsen et al., 1999), density lipoprotein receptor (LDLR) and caveolin-1 (Cav-1). -

Caveolin-1 Is Down-Regulated in Alveolar Rhabdomyosarcomas and Negatively Regulates Tumor Growth
www.impactjournals.com/oncotarget/ Oncotarget, Vol. 5, No. 20 Caveolin-1 is down-regulated in alveolar rhabdomyosarcomas and negatively regulates tumor growth Juan Huertas-Martínez1, Santiago Rello-Varona1, David Herrero-Martín1, Ignasi Barrau1, Silvia García-Monclús1, Miguel Sáinz-Jaspeado1, Laura Lagares-Tena1, Yaiza Núñez-Álvarez5, Silvia Mateo-Lozano2, Jaume Mora2, Josep Roma3, Nuria Toran3, Sebastian Moran4, Roser López-Alemany1, Soledad Gallego3, Manel Esteller4, Miguel A. Peinado5, Xavier García del Muro1 and Oscar M. Tirado1 1 Sarcoma research group, Molecular Oncology Lab, Bellvitge Biomedical Research Institute (IDIBELL), L’Hospitalet de Llobregat, Barcelona, Spain 2 Developmental Tumor Biology Laboratory, Hospital Sant Joan de Deu, Barcelona, Spain 3 Biomedical Research Unit, Hospital Universitari Vall d’Hebron, Barcelona, Spain 4 Cancer Epigenetics and Biology Programme (PEBC), Bellvitge Biomedical Research Institute (IDIBELL), L’ Hospitalet de Llobregat, Barcelona, Spain 5 Institut de Medicina Predictiva i Personalitzada del Càncer, Badalona, Barcelona, Spain Correspondence to: Oscar M. Tirado, email: [email protected] Keywords: alveolar rhabdomyosarcoma, Caveolin-1, muscular differentiation, 5-AZA-2’-deoxycytidine, epigenetics, cell death Received: June 26, 2014 Accepted: August 26, 2014 Published: August 27, 2014 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT Rhabdomyosarcoma is the most common soft tissue sarcoma of childhood and adolescence. Despite advances in therapy, patients with histological variant of rhabdomyosarcoma known as alveolar rhabdomyosarcoma (ARMS) have a 5-year survival of less than 30%. Caveolin-1 (CAV1), encoding the structural component of cellular caveolae, is a suggested tumor suppressor gene involved in cell signaling. -

Role of Stromal Caveolin-1 (CAV1) Levels in Breast Cancer Angiogenesis
Universidad Autónoma de Madrid Programa de Doctorado en Biociencias Moleculares Role of stromal Caveolin-1 (CAV1) levels in breast cancer angiogenesis Alberto Díez Sánchez Madrid, 2018 0 1 Departamento de Bioquímica Facultad de Medicina Universidad Autónoma de Madrid Role of stromal Caveolin-1 (CAV1) levels in breast cancer angiogenesis Doctorando: Alberto Díez Sánchez, Licenciado en Biotecnología Director: Miguel Ángel del Pozo Barriuso, MD, PhD. Fundación Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC) Madrid, 2018 1 2 CERTIFICADO DEL DIRECTOR DE TESIS El doctor Miguel Ángel del Pozo Barriuso CERTIFICA que el doctorando Alberto Díez Sánchez ha desarrollado y concluido su trabajo de tesis doctoral “Role of stromal Caveolin-1 (CAV1) levels in breast cancer angiogenesis” bajo su supervisión, en el Centro Nacional de Investigaciones Cardiovasculares (CNIC). Y para que así conste lo firma en Madrid, a 10 de Julio de 2018, Fdo. Dr. Miguel Ángel del Pozo Barriuso Centro Nacional de Investigaciones Cardiovasculares (CNIC) 3 4 ACKNOWLEDGMENTS It is said that scientific knowledge is built on top of the shoulder of giants, in more practical terms, I consider all these people below my personal giants. First ones I encountered, were my parents and grandparents, everything I have achieved has been done on top of their previous efforts, to them I dedicate my most sincere gratitude for teaching this once lazy kid the value of effort. Next, I have to thank all those high-school teachers and university professors that during my education have been able to spark in me the sense of amazement derived from understanding how nature works. -

Novel Missense Mutation in the Caveolin-3 Gene in a Belgian Family
1349 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2003.028217 on 16 August 2004. Downloaded from SHORT REPORT Novel missense mutation in the caveolin-3 gene in a Belgian family with rippling muscle disease P Y K Van den Bergh, J M Ge´rard, J A Elosegi, M U Manto, C Kubisch, B G H Schoser ............................................................................................................................... J Neurol Neurosurg Psychiatry 2004;75:1349–1351. doi: 10.1136/jnnp.2003.028217 too small’’). The patient was diagnosed as having fibromyal- Rippling muscle disease (RMD) is a rare muscle disorder gia, which led to secondary depression and loss of her job. characterised by muscle stiffness, exercise induced myalgia, Muscle weakness and pigmenturia were absent. The medical and cramp-like sensations. It is genetically heterogeneous history was remarkable for mild hypothyroidism, peptic and can be acquired, but most cases show autosomal oesophagitis, and tobacco related asthmatiform bronchitis dominant inheritance due to mutations in the caveolin-3 with emphysema. At age 38, she had a neurological work-up. (CAV3) gene. We report a novel heterozygous missense The cranial nerves, muscle bulk, muscle strength, and deep mutation in CAV3 in a Belgian family with autosomal tendon reflexes were normal, and plantar responses were dominant RMD. flexor. Tapping with the reflex hammer or with the finger on A 40 year old woman complained of fatigue, exercise the muscles provoked an immediate, short lasting, forceful induced muscle pain, and muscle cramps since the age of 35. contraction and/or painful local mounding lasting for several Neurological examination revealed percussion induced seconds. PIRCs were most pronounced in the sternocleido- rapid muscle contractions (PIRCs) and localised muscle mastoid, deltoid, biceps, brachioradialis, finger and wrist mounding on percussion; muscle rippling was not observed. -

The Popeye Domain Containing Genes and Their Function in Striated Muscle
Journal of Cardiovascular Development and Disease Review The Popeye Domain Containing Genes and Their Function in Striated Muscle Roland F. R. Schindler 1, Chiara Scotton 2, Vanessa French 1, Alessandra Ferlini 2 and Thomas Brand 1,* 1 Developmental Dynamics, Harefield Heart Science Centre, National Heart and Lung Institute, Imperial College London, Hill End Road, Harefield UB9 6JH, UK; [email protected] (R.F.R.S.); [email protected] (V.F.) 2 Department of Medical Sciences, Medical Genetics Unit, University of Ferrara, Ferrara 44121, Italy; [email protected] (C.S.); fl[email protected] (A.F.) * Correspondence: [email protected]; Tel.: +44-189-582-8900 Academic Editors: Robert E. Poelmann and Monique R.M. Jongbloed Received: 26 April 2016; Accepted: 13 June 2016; Published: 15 June 2016 Abstract: The Popeye domain containing (POPDC) genes encode a novel class of cAMP effector proteins, which are abundantly expressed in heart and skeletal muscle. Here, we will review their role in striated muscle as deduced from work in cell and animal models and the recent analysis of patients carrying a missense mutation in POPDC1. Evidence suggests that POPDC proteins control membrane trafficking of interacting proteins. Furthermore, we will discuss the current catalogue of established protein-protein interactions. In recent years, the number of POPDC-interacting proteins has been rising and currently includes ion channels (TREK-1), sarcolemma-associated proteins serving functions in mechanical stability (dystrophin), compartmentalization (caveolin 3), scaffolding (ZO-1), trafficking (NDRG4, VAMP2/3) and repair (dysferlin) or acting as a guanine nucleotide exchange factor for Rho-family GTPases (GEFT). -
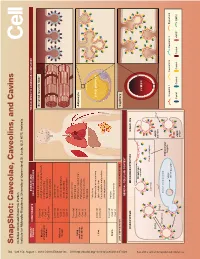
Snapshot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G
704 Cell SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton 154 Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia , August 1,2013©2013 Elsevier Inc. DOI http://dx.doi.org/10.1016/j.cell.2013.07.009 ORGAN/ COMPONENTS DISEASE AND TISSUE-SPECIFIC CAVEOLAR COMPLEXES TISSUE CELLULAR PROCESS Rippling muscle disease Caveolin3 Striated muscle fiber Limb girdle muscular dystrophy Muscle Cavin1 skeletal and Cardiomyopathy Cavin4 cardiac Mechanoprotection Cav1 (heart) T-tubule formation Caveolin1 Lipodystrophy Caveolin2 Lipotoxicity Adipose Cavin1 Fatty acid regulation tissue Cavin2 Insulin signaling Cavin3 Mechanoprotection Atherosclerosis Caveolin1 Inammation Caveolin2 Lung Pulmonary hypertension and other Cavin1 Adipocyte Pulmonary brosis endothelia Cavin2 Mechanosensation Cavin3 Signaling Fatty liver Hepatocellular carcinoma Caveolin1 LIPID DROPLET Liver Lipid metabolism Caveolin2 Carbohydrate metabolism Liver regeneration Caveolin1 Autism Brain Cavin1 Schizophrenia Cavin3 LOW EXPRESSION HIGH EXPRESSION Capillary GENERAL CELLULAR CONTEXT ENDOCYTOSIS MECHANOPROTECTION SIGNALING See online version for legend and references. and legend for version online See Membrane stretch LUMEN Cavin complex Caveola EHD2 eNOS Ca2+ inactive Rab5 Dynamin2 EARLY ENDOSOME Intracellular targets MVB/ Caveolin 1 Caveolin 2 Caveolin 3 Dynamin2 late endosome eNOS Cavin1 Cavin2 Cavin3 Cavin4 eNOS EHD2 active SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia Caveolae, submicroscopic bulb-shaped plasma membrane pits, are an abundant feature of many mammalian cells (Parton and del Pozo, 2013). Caveolae and the major proteins of caveolae, caveolins (Rothberg et al., 1992), and cavins (Hill et al., 2008), are linked to a number of human diseases such as muscular dystrophy, cardiomyopathy, and lipodys- trophy (see Glossary) (Bruno et al., 1993; Fernández et al., 2006; Hayashi et al., 2009). -

Development of an Infectious Clone System to Study the Life Cycle of Hazara Virus
Development of an Infectious Clone System to Study the Life Cycle of Hazara Virus Jack Fuller Submitted in accordance with the requirements for the degree of Doctor of Philosophy The University of Leeds Faculty of Biological Sciences July 2020 i The candidate confirms that the work submitted is his own and that appropriate credit has been given where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. © 2020 The University of Leeds and Jack Fuller ii Acknowledgements I would like to thank my supervisors, John, Jamel and Roger for their endless support and fresh ideas throughout my PhD. A special thanks go to John for always providing enthusiasm and encouragement, especially during the tougher times of the project! I would also like to thank everyone in 8.61, not only for making my time at Leeds enjoyable, but for helping me develop as a scientist through useful critique and discussion. A special thanks goes to Francis Hopkins, for welcoming me into the Barr group and providing a constant supply of humour and to Ellie Todd, for listening to my endless whines and gripes, and for providing welcome distractions in the form of her PowerPoint artwork. Outside of the lab, my mum deserves a special mention for always being the voice of reason and support at the end of the phone, but also for pushing me to succeed throughout my entire education. I have no doubts I would not be in the position I am now without her! Finally, a huge thanks to my partner Hannah, who has been there to support me through all the highs and lows of my PhD, and has sacrificed many weekend trips to allow me to finish experiments and to tend to my viruses! iii Abstract Crimean-Congo hemorrhagic fever orthonairovirus (CCHFV) is a negative sense single stranded RNA virus, capable of causing fatal hemorrhagic fever in humans. -

Caveolar Endocytosis of Simian Virus 40 Reveals a New Two-Step Vesicular- Transport Pathway to the ER
articles Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular- transport pathway to the ER Lucas Pelkmans*, Jürgen Kartenbeck† and Ari Helenius*‡ *Institute of Biochemistry, Swiss Federal Institute of Technology, Universitaetstrasse 16, CH-8092 Zürich, Switzerland †German Cancer Research Center (DKFZ) Heidelberg, Im Neuenheimer Feld 280, D-69120 Heidelberg, Germany ‡e-mail: [email protected] Simian virus 40 (SV40) is unusual among animal viruses in that it enters cells through caveolae, and the internalized virus accumulates in a smooth endoplasmic reticulum (ER) compartment. Using video-enhanced, dual-colour, live fluorescence microscopy, we show the uptake of individual virus particles in CV-1 cells. After associating with cave- olae, SV40 leaves the plasma membrane in small, caveolin-1-containing vesicles. It then enters larger, peripheral organelles with a non-acidic pH. Although rich in caveolin-1, these organelles do not contain markers for endo- somes, lysosomes, ER or Golgi, nor do they acquire ligands of clathrin-coated vesicle endocytosis. After several hours in these organelles, SV40 is sorted into tubular, caveolin-free membrane vesicles that move rapidly along microtubules, and is deposited in perinuclear, syntaxin 17-positive, smooth ER organelles. The microtubule-disrupt- ing agent nocodazole inhibits formation and transport of these tubular carriers, and blocks viral infection. Our results demonstrate the existence of a two-step transport pathway from plasma-membrane caveolae, through an intermediate organelle (termed the caveosome), to the ER. This pathway bypasses endosomes and the Golgi com- plex, and is part of the productive infectious route used by SV40. any animal viruses take advantage of receptor-mediated mutants of caveolin-3 localize to intracellular vesicles that are dis- endocytosis to enter their host cells. -

Starting a Molecular Systems View of Endocytosis
ANRV356-CB24-20 ARI 3 September 2008 19:11 ANNUAL Protein Kinases: Starting REVIEWS Further Click here for quick links to Annual Reviews content online, a Molecular Systems View including: • Other articles in this volume of Endocytosis • Top cited articles • Top downloaded articles • Our comprehensive search Prisca Liberali, Pauli Ram¨ o,¨ and Lucas Pelkmans Institute of Molecular Systems Biology, ETH Zurich, CH-8093 Zurich, Switzerland; email: [email protected] Annu. Rev. Cell Dev. Biol. 2008. 24:501–23 Key Words First published online as a Review in Advance on membrane trafficking, phosphorylation, signal transduction, July 3, 2008 complexity, nonlinear systems, genetical physics The Annual Review of Cell and Developmental Biology is online at cellbio.annualreviews.org Abstract This article’s doi: The field of endocytosis is in strong need of formal biophysical model- 10.1146/annurev.cellbio.041008.145637 ing and mathematical analysis. At the same time, endocytosis must be Copyright c 2008 by Annual Reviews. much better integrated into cellular physiology to understand the for- by Universitat Zurich- Hauptbibliothek Irchel on 04/05/13. For personal use only. All rights reserved mer’s complex behavior in such a wide range of phenotypic variations. Annu. Rev. Cell Dev. Biol. 2008.24:501-523. Downloaded from www.annualreviews.org 1081-0706/08/1110-0501$20.00 Furthermore, the concept that endocytosis provides the space-time for signal transduction can now be experimentally addressed. In this review, we discuss these principles and argue for a systematic and top-down ap- proach to study the endocytic membrane system. We provide a summary of published observations on protein kinases regulating endocytic ma- chinery components and discuss global unbiased approaches to further map out kinase regulatory networks. -
Modulation of the Caveolin-3 Localization to Caveolae and STAT3 to Mitochondria by Catecholamine-Induced Cardiac Hypertrophy in H9c2 Cardiomyoblasts
EXPERIMENTAL and MOLECULAR MEDICINE, Vol. 41, No. 4, 226-235, April 2009 Modulation of the caveolin-3 localization to caveolae and STAT3 to mitochondria by catecholamine-induced cardiac hypertrophy in H9c2 cardiomyoblasts Kyuho Jeong*, Hayeong Kwon*, amine-induced cardiac hypertrophy. Chanhee Min and Yunbae Pak1 Keywords: cardiomegaly; caveolae; caveolin-3; cell Department of Biochemistry nucleus; heart; isoproterenol; mitochondria; phenyl- Division of Applied Life Science (BK21), PMBBRC ephrine; STAT3 transcription factor Environmental Biotechnology National Core Research Center Gyeongsang National University Jinju 660-701, Korea Introduction 1Corresponding author: Tel, 82-55-751-5961; Fax, 82-55-759-9363; E-mail, [email protected] Hypertension is major risk factors for cardiac da- mage, ischemia, myocardial infarction, and conge- *These authors contributed equally to this work. stive heart failure (Zampaglione et al., 1996). In DOI 10.3858/emm.2009.41.4.025 response to increased demands for cardiac work caused by various pathologic stresses, heart adapts Accepted 20 November 2008 through compensatory hypertrophy of myocytes. Abbreviations: Akt, protein kinase B; CsA, cyclosporin A; GPCR, Thus, cardiac hypertrophy is recognized in many G protein-coupled receptor; ISO, isoproterenol; PE, phenylephrine; cardiovascular diseases, such as hypertension, RTK, receptor tyrosine kinase; STAT3, signal transducers and acti- vascular disease, and myocardial infarction, and is vator of transcription 3 an independent risk factor for cardiac morbidity and mortality. Hypertrophic stimuli induce an in- crease in cell size in the absence of cell division through Ca2+ signaling and activation of PKC, Abstract MAPK and PKB/ Akt (Watanabe et al., 2001; Dorn and Force, 2005), and are accompanied by We investigated the effect of phenylephrine (PE)- and increased protein synthesis with reprogramming of isoproterenol (ISO)-induced cardiac hypertrophy on gene expression (Takeo et al., 2000).