Internalization of the TGF-Β Type I Receptor Into Caveolin-1 and EEA1 Double-Positive Early Endosomes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abl Family Tyrosine Kinases Govern Igg Extravasation in the Skin in a Murine Pemphigus Model
ARTICLE https://doi.org/10.1038/s41467-019-12232-3 OPEN Abl family tyrosine kinases govern IgG extravasation in the skin in a murine pemphigus model Sachiko Ono1, Gyohei Egawa1, Takashi Nomura1, Akihiko Kitoh1, Teruki Dainichi 1, Atsushi Otsuka1, Saeko Nakajima1, Masayuki Amagai2, Fumi Matsumoto3, Mami Yamamoto 3, Yoshiaki Kubota4, Toshiyuki Takai5, Tetsuya Honda1 & Kenji Kabashima 1,6 1234567890():,; The pathway of homeostatic IgG extravasation is not fully understood, in spite of its importance for the maintenance of host immunity, the management of autoantibody- mediated disorders, and the use of antibody-based biologics. Here we show in a murine model of pemphigus, a prototypic cutaneous autoantibody-mediated disorder, that blood- circulating IgG extravasates into the skin in a time- and dose-dependent manner under homeostatic conditions. This IgG extravasation is unaffected by depletion of Fcγ receptors, but is largely attenuated by specific ablation of dynamin-dependent endocytic vesicle for- mation in blood endothelial cells (BECs). Among dynamin-dependent endocytic vesicles, IgG co-localizes well with caveolae in cultured BECs. An Abl family tyrosine kinase inhibitor imatinib, which reduces caveolae-mediated endocytosis, impairs IgG extravasation in the skin and attenuates the murine pemphigus manifestations. Our study highlights the kinetics of IgG extravasation in vivo, which might be a clue to understand the pathological mechanism of autoantibody-mediated autoimmune disorders. 1 Department of Dermatology, Kyoto University Graduate School of Medicine, Kyoto, Japan. 2 Department of Dermatology, Keio University Graduate School of Medicine, Tokyo, Japan. 3 Research Unit/Immunology & Inflammation, Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, Yokohama, Japan. 4 Department of Anatomy, Keio University School of Medicine, Tokyo, Japan. -

An Arf1 Synthetic Lethal Screen Identifies a New Clathrin Heavy
Copyright 1998 by the Genetics Society of America An arf1D Synthetic Lethal Screen Identi®es a New Clathrin Heavy Chain Conditional Allele That Perturbs Vacuolar Protein Transport in Saccharomyces cerevisiae Chih-Ying Chen and Todd R. Graham Department of Molecular Biology, Vanderbilt University, Nashville, Tennessee 37235 Manuscript received March 5, 1998 Accepted for publication June 16, 1998 ABSTRACT ADP-ribosylation factor (ARF) is a small GTP-binding protein that is thought to regulate the assembly of coat proteins on transport vesicles. To identify factors that functionally interact with ARF, we have performed a genetic screen in Saccharomyces cerevisiae for mutations that exhibit synthetic lethality with an arf1D allele and de®ned seven genes by complementation tests (SWA1-7 for synthetically lethal with arf1D). Most of the swa mutants exhibit phenotypes comparable to arf1D mutants such as temperature-conditional growth, hypersensitivity to ¯uoride ions, and partial protein transport and glycosylation defects. Here, we report that swa5-1 is a new temperature-sensitive allele of the clathrin heavy chain gene (chc1-5), which carries a frameshift mutation near the 39 end of the CHC1 open reading frame. This genetic interaction between arf1 and chc1 provides in vivo evidence for a role for ARF in clathrin coat assembly. Surprisingly, strains harboring chc1-5 exhibited a signi®cant defect in transport of carboxypeptidase Y or carboxypepti- dase S to the vacuole that was not observed in other chc1 ts mutants. The kinetics of invertase secretion or transport of alkaline phosphatase to the vacuole were not signi®cantly affected in the chc1-5 mutant, further implicating clathrin speci®cally in the Golgi to vacuole transport pathway for carboxypeptidase Y. -

The Mir-199–Dynamin Regulatory Axis Controls Receptor-Mediated Endocytosis Juan F
© 2015. Published by The Company of Biologists Ltd | Journal of Cell Science (2015) 128, 3197-3209 doi:10.1242/jcs.165233 RESEARCH ARTICLE The miR-199–dynamin regulatory axis controls receptor-mediated endocytosis Juan F. Aranda1,2, Alberto Canfrán-Duque1,2, Leigh Goedeke1,2, Yajaira Suárez1,2 and Carlos Fernández-Hernando1,2,* ABSTRACT mechanism for the selective uptake of essential nutrients such as Small non-coding RNAs (microRNAs) are important regulators of low-density lipoprotein (LDL), through the LDL receptor (LDLR) gene expression that modulate many physiological processes; (Brown and Goldstein, 1986), or iron, through transferrin receptor however, their role in regulating intracellular transport remains (TfR) (Harding et al., 1983). Thus, factors that affect RME have a largely unknown. Intriguingly, we found that the dynamin (DNM) direct effect on these receptors, and, in the case of LDLR, to regulate genes, a GTPase family of proteins responsible for endocytosis in intracellular cholesterol levels. In both the LDLR and TfR eukaryotic cells, encode the conserved miR-199a and miR-199b internalization processes, clathrin plays a key role during the family of miRNAs within their intronic sequences. Here, we formation of coated vesicles (Moore et al., 1987). Once vesicles are demonstrate that miR-199a and miR-199b regulate endocytic internalized, their passage through a broad endosomal compartment transport by controlling the expression of important mediators of system is required; first they are rapidly transported into early endocytosis such as clathrin heavy chain (CLTC), Rab5A, low- endosomes, where Rab5A is a key regulator (Nielsen et al., 1999), density lipoprotein receptor (LDLR) and caveolin-1 (Cav-1). -

ADP-Ribosylation Factor, a Small GTP-Binding Protein, Is Required for Binding of the Coatomer Protein Fl-COP to Golgi Membranes JULIE G
Proc. Natl. Acad. Sci. USA Vol. 89, pp. 6408-6412, July 1992 Biochemistry ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein fl-COP to Golgi membranes JULIE G. DONALDSON*, DAN CASSEL*t, RICHARD A. KAHN*, AND RICHARD D. KLAUSNER* *Cell Biology and Metabolism Branch, National Institute of Child Health and Human Development, and tLaboratory of Biological Chemistry, Division of Cancer Treatment, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892 Communicated by Marc Kirschner, April 20, 1992 (receivedfor review February 11, 1992) ABSTRACT The coatomer is a cytosolic protein complex localized to the Golgi complex, although their functions have that reversibly associates with Golgi membranes and is Impli- not been defined. Distinct among these proteins is the ADP- cated in modulating Golgi membrane transport. The associa- ribosylation factor (ARF), originally identified as a cofactor tion of 13-COP, a component of coatomer, with Golgi mem- required for in vitro cholera toxin-catalyzed ADP- branes is enhanced by guanosine 5'-[v-thioltriphosphate ribosylation of the a subunit of the trimeric GTP-binding (GTP[yS]), a nonhydrolyzable analogue of GTP, and by a protein G, (G,.) (19). ARF is an abundant cytosolic protein mixture of aluminum and fluoride ions (Al/F). Here we show that reversibly associates with Golgi membranes (20, 21). that the ADP-ribosylation factor (ARF) is required for the ARF has been shown to be present on Golgi coated vesicles binding of (-COP. Thus, 13-COP contained in a coatomer generated in the presence of GTP[yS], but it is not a com- fraction that has been resolved from ARF does not bind to Golgi ponent of the cytosolic coatomer (22). -

Palmitoylation of Caveolin-1 and Its Importance for Structural and Functional Plasticity
Palmitoylation of Caveolin-1 and its importance for structural and functional plasticity A DISSERTATION SUBMITTED TO THE FACULTY OF UNIVERSITY OF MINNESOTA BY Katherine R. Tonn IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Paul G. Mermelstein November 2018 © Katherine R. Tonn Eisinger 2018 Acknowledgments I would like to thank my adviser, Dr. Paul Mermelstein, for his mentorship, training, support, and scientific flexibility. Infinite thanks to past and present members of the Mermelstein and Meisel Labs, especially Dr. Brittni Peterson, Dr. Kelsey Moore, Dr. Laura Been, Dr. Luis Martinez, Dr. Valerie Hedges, and Dr. John Meitzen, for being a source of scientific inspiration and friendship. I could not have asked for a better friend and lab colleague than Dr. Kellie Gross, whose input has kept me sane and made me a better scientist. Thanks also to stellar undergrads Kerry Trotter, Julia Dworsky, and Sam Swanson for their help and energy. Thanks to the many people who collaborated or helped in some way on the experiments presented in this thesis, including Dr. Mark Thomas, Dr. Lorene Lanier, Dr. Mark Dell’Acqua, Dr. Kevin Woolfrey, Dr. Brian Head, Dr. Jing Tong, and Dr. John Meitzen. In particular, I am extremely grateful for the collaboration of Dr. Lorene Lanier, whose enthusiasm helped reinvigorate me and this project. Finally, thanks to the members of my thesis committee, Drs. Harry Orr, Timothy Ebner, Anna Lee, and Robert Meisel, for their encouragement and guidance. i Dedication For Mom, my first and most important example of what it is to be smart, versatile, and kind. -

Role of Stromal Caveolin-1 (CAV1) Levels in Breast Cancer Angiogenesis
Universidad Autónoma de Madrid Programa de Doctorado en Biociencias Moleculares Role of stromal Caveolin-1 (CAV1) levels in breast cancer angiogenesis Alberto Díez Sánchez Madrid, 2018 0 1 Departamento de Bioquímica Facultad de Medicina Universidad Autónoma de Madrid Role of stromal Caveolin-1 (CAV1) levels in breast cancer angiogenesis Doctorando: Alberto Díez Sánchez, Licenciado en Biotecnología Director: Miguel Ángel del Pozo Barriuso, MD, PhD. Fundación Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC) Madrid, 2018 1 2 CERTIFICADO DEL DIRECTOR DE TESIS El doctor Miguel Ángel del Pozo Barriuso CERTIFICA que el doctorando Alberto Díez Sánchez ha desarrollado y concluido su trabajo de tesis doctoral “Role of stromal Caveolin-1 (CAV1) levels in breast cancer angiogenesis” bajo su supervisión, en el Centro Nacional de Investigaciones Cardiovasculares (CNIC). Y para que así conste lo firma en Madrid, a 10 de Julio de 2018, Fdo. Dr. Miguel Ángel del Pozo Barriuso Centro Nacional de Investigaciones Cardiovasculares (CNIC) 3 4 ACKNOWLEDGMENTS It is said that scientific knowledge is built on top of the shoulder of giants, in more practical terms, I consider all these people below my personal giants. First ones I encountered, were my parents and grandparents, everything I have achieved has been done on top of their previous efforts, to them I dedicate my most sincere gratitude for teaching this once lazy kid the value of effort. Next, I have to thank all those high-school teachers and university professors that during my education have been able to spark in me the sense of amazement derived from understanding how nature works. -

Dynamin Action at the Final Stage of Clathrin- Mediated Endocytosis
Dynamin Action at the Final Stage of Clathrin- Mediated Endocytosis Ning Fang ( [email protected] ) Georgia State University https://orcid.org/0000-0003-4710-0984 Xiaodong Cheng Georgia State University Kuangcai Chen Georgia State University https://orcid.org/0000-0002-9321-9225 Bin Dong Georgia State University https://orcid.org/0000-0002-3196-0712 Meek Yang Georgia State University Seth Filbrun Georgia State University Yong Myoung Georgia State University Teng-Xiang Huang Georgia State University Yan Gu The Bristol-Myers Squibb Company Gufeng Wang Georgia State University Article Keywords: Dynamin, clathrin-mediated endocytosis, scission, vesicle ssion Posted Date: February 5th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-134570/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/25 Abstract Dynamin plays an important role in clathrin-mediated endocytosis by cutting the neck of nascent vesicles from the cell membrane. Gold nanorods were used as imaging probes to observe dynamin action on cargo vesicles during live endocytosis events. Invariant is that at the peak of dynamin accumulation, the cargo-containing vesicle always gives abrupt, right-handed rotations that nishes in a short time (~ 0.28 s). The large and quick twist, herein named the super twist, is the result of the coordinated dynamin helix action upon GTP hydrolysis. After the super twist, the rotational freedom of the vesicle drastically increases, accompanied with simultaneous or delayed translational movement, indicating that it detaches from the cell membrane. These observations suggest that dynamin-mediated scission at the nal stage involves a large torque generated by coordinated actions of multiple dynamins in the helix, which is the main driving force for scission. -

Novel Missense Mutation in the Caveolin-3 Gene in a Belgian Family
1349 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2003.028217 on 16 August 2004. Downloaded from SHORT REPORT Novel missense mutation in the caveolin-3 gene in a Belgian family with rippling muscle disease P Y K Van den Bergh, J M Ge´rard, J A Elosegi, M U Manto, C Kubisch, B G H Schoser ............................................................................................................................... J Neurol Neurosurg Psychiatry 2004;75:1349–1351. doi: 10.1136/jnnp.2003.028217 too small’’). The patient was diagnosed as having fibromyal- Rippling muscle disease (RMD) is a rare muscle disorder gia, which led to secondary depression and loss of her job. characterised by muscle stiffness, exercise induced myalgia, Muscle weakness and pigmenturia were absent. The medical and cramp-like sensations. It is genetically heterogeneous history was remarkable for mild hypothyroidism, peptic and can be acquired, but most cases show autosomal oesophagitis, and tobacco related asthmatiform bronchitis dominant inheritance due to mutations in the caveolin-3 with emphysema. At age 38, she had a neurological work-up. (CAV3) gene. We report a novel heterozygous missense The cranial nerves, muscle bulk, muscle strength, and deep mutation in CAV3 in a Belgian family with autosomal tendon reflexes were normal, and plantar responses were dominant RMD. flexor. Tapping with the reflex hammer or with the finger on A 40 year old woman complained of fatigue, exercise the muscles provoked an immediate, short lasting, forceful induced muscle pain, and muscle cramps since the age of 35. contraction and/or painful local mounding lasting for several Neurological examination revealed percussion induced seconds. PIRCs were most pronounced in the sternocleido- rapid muscle contractions (PIRCs) and localised muscle mastoid, deltoid, biceps, brachioradialis, finger and wrist mounding on percussion; muscle rippling was not observed. -

Membrane Remodeling in Clathrin-Mediated Endocytosis Volker Haucke1,2,* and Michael M
© 2018. Published by The Company of Biologists Ltd | Journal of Cell Science (2018) 131, jcs216812. doi:10.1242/jcs.216812 REVIEW Membrane remodeling in clathrin-mediated endocytosis Volker Haucke1,2,* and Michael M. Kozlov3,* ABSTRACT membrane fission and vesicle uncoating to eventually allow fusion Clathrin-mediated endocytosis is an essential cellular mechanism by of the nascent endocytic vesicle with endosomes (Box 1). which all eukaryotic cells regulate their plasma membrane composition In spite of important advances in the characterization of these to control processes ranging from cell signaling to adhesion, migration factors, key questions regarding the endocytic process remain. and morphogenesis. The formation of endocytic vesicles and For example, different models have been proposed regarding the tubules involves extensive protein-mediated remodeling of the initiation of clathrin-coated pit (CCP) formation and the onset of plasma membrane that is organized in space and time by protein– membrane curvature acquisition. Moreover, knowledge regarding protein and protein–phospholipid interactions. Recent studies the nanoscale distribution of endocytic proteins within the bilayer combining high-resolution imaging with genetic manipulations of plane during vesicle formation and the mechanisms that guide the endocytic machinery and with theoretical approaches have led their orchestrated assembly and disassembly is only now beginning to novel multifaceted phenomenological data of the temporal and to emerge. Finally, our understanding of the nature and dynamics spatial organization of the endocytic reaction. This gave rise to of membrane phospholipids (Box 2) and their associations with various – often conflicting – models as to how endocytic proteins endocytic proteins during endocytic membrane remodeling and their association with lipids regulate the endocytic protein remains limited. -

Cryo-Tomography of Coat Complexes ISSN 2059-7983
research papers Visualizing membrane trafficking through the electron microscope: cryo-tomography of coat complexes ISSN 2059-7983 Evgenia A. Markova and Giulia Zanetti* Institute of Structural and Molecular Biology, Birkbeck College, Malet Street, London WC1E 7HX, England. Received 8 March 2019 *Correspondence e-mail: [email protected] Accepted 12 April 2019 Coat proteins mediate vesicular transport between intracellular compartments, Keywords: cryo-electron tomography; which is essential for the distribution of molecules within the eukaryotic cell. three-dimensional reconstruction; coat proteins; The global arrangement of coat proteins on the membrane is key to their vesicular transport; COPII; subtomogram function, and cryo-electron tomography and subtomogram averaging have been averaging. used to study membrane-bound coat proteins, providing crucial structural insight. This review outlines a workflow for the structural elucidation of coat proteins, incorporating recent developments in the collection and processing of cryo-electron tomography data. Recent work on coat protein I, coat protein II and retromer performed on in vitro reconstitutions or in situ is summarized. These studies have answered long-standing questions regarding the mechanisms of membrane binding, polymerization and assembly regulation of coat proteins. 1. Introduction Recent advances in cryo-electron microscopy (cryo-EM) have enabled numerous high-resolution studies which have addressed long-standing structural biology questions. The large body of cryo-EM work carried out for protein structure characterization has utilized single-particle electron micro- scopy (Cheng, 2018). Recently, developments in hardware, data collection and data processing have placed cryo-electron tomography (cryo-ET) and subtomogram averaging (STA) at the forefront of structural studies of repetitive assemblies, reaching resolutions comparable to those of single-particle EM (Schur et al., 2016; Wan et al., 2017; Hutchings et al., 2018; Dodonova et al., 2017; Himes & Zhang, 2018). -
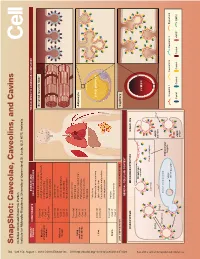
Snapshot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G
704 Cell SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton 154 Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia , August 1,2013©2013 Elsevier Inc. DOI http://dx.doi.org/10.1016/j.cell.2013.07.009 ORGAN/ COMPONENTS DISEASE AND TISSUE-SPECIFIC CAVEOLAR COMPLEXES TISSUE CELLULAR PROCESS Rippling muscle disease Caveolin3 Striated muscle fiber Limb girdle muscular dystrophy Muscle Cavin1 skeletal and Cardiomyopathy Cavin4 cardiac Mechanoprotection Cav1 (heart) T-tubule formation Caveolin1 Lipodystrophy Caveolin2 Lipotoxicity Adipose Cavin1 Fatty acid regulation tissue Cavin2 Insulin signaling Cavin3 Mechanoprotection Atherosclerosis Caveolin1 Inammation Caveolin2 Lung Pulmonary hypertension and other Cavin1 Adipocyte Pulmonary brosis endothelia Cavin2 Mechanosensation Cavin3 Signaling Fatty liver Hepatocellular carcinoma Caveolin1 LIPID DROPLET Liver Lipid metabolism Caveolin2 Carbohydrate metabolism Liver regeneration Caveolin1 Autism Brain Cavin1 Schizophrenia Cavin3 LOW EXPRESSION HIGH EXPRESSION Capillary GENERAL CELLULAR CONTEXT ENDOCYTOSIS MECHANOPROTECTION SIGNALING See online version for legend and references. and legend for version online See Membrane stretch LUMEN Cavin complex Caveola EHD2 eNOS Ca2+ inactive Rab5 Dynamin2 EARLY ENDOSOME Intracellular targets MVB/ Caveolin 1 Caveolin 2 Caveolin 3 Dynamin2 late endosome eNOS Cavin1 Cavin2 Cavin3 Cavin4 eNOS EHD2 active SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia Caveolae, submicroscopic bulb-shaped plasma membrane pits, are an abundant feature of many mammalian cells (Parton and del Pozo, 2013). Caveolae and the major proteins of caveolae, caveolins (Rothberg et al., 1992), and cavins (Hill et al., 2008), are linked to a number of human diseases such as muscular dystrophy, cardiomyopathy, and lipodys- trophy (see Glossary) (Bruno et al., 1993; Fernández et al., 2006; Hayashi et al., 2009). -

Comprehensive Analysis of Lncrna‑Associated Cerna Network Reveals the Novel Potential of Lncrna, Mirna and Mrna Biomarkers in Human Rectosigmoid Junction Cancer
ONCOLOGY LETTERS 21: 144, 2021 Comprehensive analysis of lncRNA‑associated ceRNA network reveals the novel potential of lncRNA, miRNA and mRNA biomarkers in human rectosigmoid junction cancer QIANSHI ZHANG1*, ZHEN FENG1*, SHASHA SHI2, YU ZHANG3 and SHUANGYI REN1 Departments of 1Gastrointestinal Surgery, 2Ultrasound and 3Clinical Laboratory, The Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning 116023, P.R. China Received March 11, 2020; Accepted November 20, 2020 DOI: 10.3892/ol.2020.12405 Abstract. Although accumulating evidence has confirmed gene 20) and three mRNAs (sodium‑ and chloride‑dependent the potential biological functions of long non‑coding RNAs taurine transporter, fibroblast growth factor 13 and tubulin (lncRNAs) as competitive endogenous RNAs (ceRNAs) in polyglutamylase TTLL7) were significantly associated with colorectal tumorigenesis and progression, few studies have OS (P<0.05). Additionally, two lncRNAs (KCNQ1OT1 focused on rectosigmoid junction cancer. In the present and MIR17HG) interacted with most of the DEmiRNAs. study, a comprehensive analysis was conducted to explore Notably, two top‑ranked miRNAs (hsa‑miR‑374a‑5p and lncRNA‑mediated ceRNA implications and their potential hsa‑miR‑374b‑5p) associated networks were identified to be value for prognosis. lncRNA, microRNA (miR/miRNA) markedly associated with the pathogenesis. Furthermore, four and mRNA expression profiles were downloaded from DEmRNAs (caveolin‑1, MET, filamin‑A and AKT3) were The Cancer Genome Atlas database. Subsequently, a enriched in the Kyoto Encylopedia of Gene and Genomes lncRNA‑miRNA‑mRNA regulatory network was constructed pathway analysis, as well as being included in the ceRNA to evaluate the functions of these differentially expressed genes network. In summary, the present results revealed that a on overall survival (OS) for rectosigmoid junction cancer.