The Role of Cox20 in Cox2 Maturation and Cytochrome C Oxidase Assembly
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
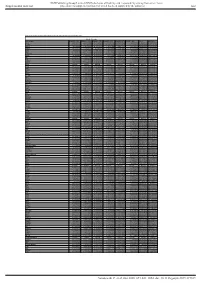
Supl Table 1 for Pdf.Xlsx
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Gut Table S1. Proteomic analysis of liver tissues from 4-months old control and LPTENKO mice. Soluble Fraction Gene names LOG2FC CTL1 LOG2FC CTL2 LOG2FC CTL3 LOG2FC KO1 LOG2FC KO2 LOG2FC KO3 t-test adj Pvalue Aass 0.081686519 -0.097912098 0.016225579 -1.135271836 -0.860535624 -1.263037541 0.0011157 1.206072068 Acsm5 -0.125220746 0.071090866 0.054129881 -0.780692107 -0.882155692 -1.158378647 0.00189031 1.021713016 Gstm2 -0.12707966 0.572554941 -0.445475281 1.952813994 1.856518122 1.561376671 0.00517664 1.865316016 Gstm1 -0.029147714 0.298593425 -0.26944571 0.983159098 0.872028945 0.786125509 0.00721356 1.949464926 Fasn 0.08403202 -0.214149498 0.130117478 1.052480559 0.779734519 1.229308218 0.00383637 0.829422353 Upb1 -0.112438784 -0.137014769 0.249453553 -1.297732445 -1.181999331 -1.495303666 0.00102373 0.184442034 Selenbp2 0.266508271 0.084791964 -0.351300235 -2.040647809 -2.608780781 -2.039865739 0.00107121 0.165425127 Thrsp -0.15001075 0.177999342 -0.027988592 1.54283307 1.603048661 0.927443822 0.00453549 0.612858263 Hyi 0.142635733 -0.183013091 0.040377359 1.325929636 1.119934412 1.181313897 0.00044587 0.053553518 Eci1 -0.119041811 -0.014846366 0.133888177 -0.599970385 -0.555547972 -1.191875541 0.02292305 2.477981171 Aldh1a7 -0.095682449 -0.017781922 0.113464372 0.653424862 0.931724091 1.110750381 0.00356922 0.350756574 Pebp1 -0.06292058 -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Mitochondrial Dysfunction and Its Role in Tissue-Specific Cellular Stress
Review www.cell-stress.com Mitochondrial dysfunction and its role in tissue-specific cellular stress David Pacheu-Grau1,*, Robert Rucktäschel1 and Markus Deckers1,* 1 Department of Cellular Biochemistry, University Medical Center Göttingen, Germany. * Corresponding Authors: David Pacheu-Grau, University Medical Center Göttingen, Department of Cellular Biochemistry, Humboldtallee 23, 37073 Göttingen, Germany. Phone: +49-(0)551-394571; E-mail: [email protected]; Markus Deckers, University Medical Center Göttingen, Department of Cellular Biochemistry, Humboldtallee 23, 37073 Göttingen, Germany. Phone: +49-(0)551-395983; E-mail: [email protected] ABSTRACT Mitochondrial bioenergetics require the coordination of two dif- doi: 10.15698/cst2018.07.147 ferent and independent genomes. Mutations in either genome will affect mi- Received originally: 26.04.2018 tochondrial functionality and produce different sources of cellular stress. De- in revised form: 13.06.2018, Accepted 14.06.2018, pending on the kind of defect and stress, different tissues and organs will be Published 13.07.2018. affected, leading to diverse pathological conditions. There is no curative ther- apy for mitochondrial diseases, nevertheless, there are strategies described that fight the various stress forms caused by the malfunctioning organelles. Keywords: mitochondrial dysfunction, Here, we will revise the main kinds of stress generated by mutations in mito- cellular stress, mitochondrial chondrial genes and outline several ways of fighting this stress. pathology, therapy. Abbreviations: ADOA – autosomal dominant optic atrophy, AROA – autosomal recessive optic atrophy, ARS – aminoacyl-tRNA synthetase, CL – cardiolipin, CRISPR – clustered regularly interspaced short palindromic repeats, LHON – Leber’s hereditary optic neuropathy, mt - mitochondrial OXPHOS – oxidative phosphorylation, ROS – reactive oxygen species. -

Impact of Disseminated Neuroblastoma Cells on the Identification of the Relapse-Seeding Clone
Author Manuscript Published OnlineFirst on February 22, 2017; DOI: 10.1158/1078-0432.CCR-16-2082 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Impact of disseminated neuroblastoma cells on the identification of the relapse-seeding clone M. Reza Abbasi1, Fikret Rifatbegovic1, Clemens Brunner1, Georg Mann2, Andrea Ziegler1, Ulrike Pötschger1, Roman Crazzolara3, Marek Ussowicz4, Martin Benesch5, Georg Ebetsberger-Dachs6, Godfrey C.F. Chan7, Neil Jones8, Ruth Ladenstein1,9, Inge M. Ambros1, Peter F. Ambros1,9 1CCRI, Children’s Cancer Research Institute, Vienna, Austria; 2St. Anna Children’s Hospital, Vienna, Austria; 3Department of Pediatrics, Medical University of Innsbruck, Innsbruck, Austria; 4Department of Pediatric Hematology and Oncology, Wroclaw Medical University, Wroclaw, Poland; 5Department of Pediatrics and Adolescent Medicine, Medical University of Graz, Graz, Austria; 6Department of Pediatrics, Kepler University Clinic Linz, Linz, Austria; 7Department of Pediatrics and Adolescent Medicine, University of Hong Kong, Hong Kong; 8Department of Pediatrics and Adolescent Medicine, Paracelsus Medical University, Salzburg, Austria; 9Department of Pediatrics, Medical University of Vienna, Vienna, Austria. Running title: Genomics of disseminated neuroblastoma cells Keywords: Neuroblastoma, disseminated tumor cells, bone marrow, tumor evolution, tumor heterogeneity Financial Support: St. Anna Kinderkrebsforschung; Austrian National Bank (ÖNB), Grant Nos. 15114, 16611; Austrian Science -

Exome Sequencing Reveals SCO2 Mutations in a Family Presented with Fatal Infantile Hyperthermia
Journal of Human Genetics (2013) 58, 226–228 & 2013 The Japan Society of Human Genetics All rights reserved 1434-5161/13 www.nature.com/jhg SHORT COMMUNICATION Exome sequencing reveals SCO2 mutations in a family presented with fatal infantile hyperthermia Nyamkhishig Sambuughin1, Xinyue Liu2, Sunita Bijarnia3, Tarina Wallace1, Ishwar C Verma3, Susan Hamilton4, Sheila Muldoon1, Luke J Tallon2 and Shuishu Wang5 We applied whole-exome sequencing (WES) for identification of an underlying genetic cause of a disease in a family presented with fatal infantile hyperthermia. Analysis of WES results revealed novel, deleterious compound missense mutations, Val160Ala and Pro233Thr, in the synthesis of cytochrome C oxidase 2 gene (SCO2) encoding a mitochondrial protein, Sco2, which is important for cytochrome C oxidase (COX) synthesis. Autosomal recessive mutations in SCO2 are known to be associated with COX deficiency recognized as fatal infantile cardio-encephalomyopathy (604272, OMIM). The Val160Ala and Pro233Thr mutations occurred in the conserved thioredoxin domain of Sco2 and predicted to disrupt protein folding and interaction of Sco2 with other proteins. Our results show applicability of WES in identification of disease-causing mutations and in establishing molecular diagnosis of severe, infantile onset disorder with a challenging diagnosis. Journal of Human Genetics (2013) 58, 226–228; doi:10.1038/jhg.2012.156; published online 31 January 2013 Keywords: exome sequencing; fatal infantile cardio-encephalomyopathy; malignant hyperthermia; recessive mutation; synthesis of cytochrome C oxidase 2 INTRODUCTION persistent hyperthermia in a hot environment. The marriage was non- Whole-exome sequencing (WES) has proven to be an extremely consanguineous, pregnancy was normal and neonatal complications were valuable and cost-effective tool for uncovering disease genes and denied (Supplementary Table S1). -

2014-Platform-Abstracts.Pdf
American Society of Human Genetics 64th Annual Meeting October 18–22, 2014 San Diego, CA PLATFORM ABSTRACTS Abstract Abstract Numbers Numbers Saturday 41 Statistical Methods for Population 5:30pm–6:50pm: Session 2: Plenary Abstracts Based Studies Room 20A #198–#205 Featured Presentation I (4 abstracts) Hall B1 #1–#4 42 Genome Variation and its Impact on Autism and Brain Development Room 20BC #206–#213 Sunday 43 ELSI Issues in Genetics Room 20D #214–#221 1:30pm–3:30pm: Concurrent Platform Session A (12–21): 44 Prenatal, Perinatal, and Reproductive 12 Patterns and Determinants of Genetic Genetics Room 28 #222–#229 Variation: Recombination, Mutation, 45 Advances in Defining the Molecular and Selection Hall B1 Mechanisms of Mendelian Disorders Room 29 #230–#237 #5-#12 13 Genomic Studies of Autism Room 6AB #13–#20 46 Epigenomics of Normal Populations 14 Statistical Methods for Pedigree- and Disease States Room 30 #238–#245 Based Studies Room 6CF #21–#28 15 Prostate Cancer: Expression Tuesday Informing Risk Room 6DE #29–#36 8:00pm–8:25am: 16 Variant Calling: What Makes the 47 Plenary Abstracts Featured Difference? Room 20A #37–#44 Presentation III Hall BI #246 17 New Genes, Incidental Findings and 10:30am–12:30pm:Concurrent Platform Session D (49 – 58): Unexpected Observations Revealed 49 Detailing the Parts List Using by Exome Sequencing Room 20BC #45–#52 Genomic Studies Hall B1 #247–#254 18 Type 2 Diabetes Genetics Room 20D #53–#60 50 Statistical Methods for Multigene, 19 Genomic Methods in Clinical Practice Room 28 #61–#68 Gene Interaction -

Regulation of COX Assembly and Function by Twin CX9C Proteins—Implications for Human Disease
cells Review Regulation of COX Assembly and Function by Twin CX9C Proteins—Implications for Human Disease Stephanie Gladyck 1, Siddhesh Aras 1,2, Maik Hüttemann 1 and Lawrence I. Grossman 1,2,* 1 Center for Molecular Medicine and Genetics, Wayne State University School of Medicine, Detroit, MI 48201, USA; [email protected] (S.G.); [email protected] (S.A.); [email protected] (M.H.) 2 Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, Maryland and Detroit, MI 48201, USA * Correspondence: [email protected] Abstract: Oxidative phosphorylation is a tightly regulated process in mammals that takes place in and across the inner mitochondrial membrane and consists of the electron transport chain and ATP synthase. Complex IV, or cytochrome c oxidase (COX), is the terminal enzyme of the electron transport chain, responsible for accepting electrons from cytochrome c, pumping protons to contribute to the gradient utilized by ATP synthase to produce ATP, and reducing oxygen to water. As such, COX is tightly regulated through numerous mechanisms including protein–protein interactions. The twin CX9C family of proteins has recently been shown to be involved in COX regulation by assisting with complex assembly, biogenesis, and activity. The twin CX9C motif allows for the import of these proteins into the intermembrane space of the mitochondria using the redox import machinery of Mia40/CHCHD4. Studies have shown that knockdown of the proteins discussed in this review results in decreased or completely deficient aerobic respiration in experimental models ranging from yeast to human cells, as the proteins are conserved across species. -

Role of Cytochrome C Oxidase Nuclear-Encoded Subunits in Health and Disease
Physiol. Res. 69: 947-965, 2020 https://doi.org/10.33549/physiolres.934446 REVIEW Role of Cytochrome c Oxidase Nuclear-Encoded Subunits in Health and Disease Kristýna ČUNÁTOVÁ1, David PAJUELO REGUERA1, Josef HOUŠTĚK1, Tomáš MRÁČEK1, Petr PECINA1 1Department of Bioenergetics, Institute of Physiology, Czech Academy of Sciences, Prague, Czech Republic Received February 2, 2020 Accepted September 13, 2020 Epub Ahead of Print November 2, 2020 Summary [email protected] and Tomáš Mráček, Department of Cytochrome c oxidase (COX), the terminal enzyme of Bioenergetics, Institute of Physiology CAS, Vídeňská 1083, 142 mitochondrial electron transport chain, couples electron transport 20 Prague 4, Czech Republic. E-mail: [email protected] to oxygen with generation of proton gradient indispensable for the production of vast majority of ATP molecules in mammalian Cytochrome c oxidase cells. The review summarizes current knowledge of COX structure and function of nuclear-encoded COX subunits, which may Energy demands of mammalian cells are mainly modulate enzyme activity according to various conditions. covered by ATP synthesis carried out by oxidative Moreover, some nuclear-encoded subunits possess tissue-specific phosphorylation apparatus (OXPHOS) located in the and development-specific isoforms, possibly enabling fine-tuning central bioenergetic organelle, mitochondria. OXPHOS is of COX function in individual tissues. The importance of nuclear- composed of five multi-subunit complexes embedded in encoded subunits is emphasized by recently discovered the inner mitochondrial membrane (IMM). Electron pathogenic mutations in patients with severe mitopathies. In transport from reduced substrates of complexes I and II to addition, proteins substoichiometrically associated with COX were cytochrome c oxidase (COX, complex IV, CIV) is found to contribute to COX activity regulation and stabilization of achieved by increasing redox potential of individual the respiratory supercomplexes. -

Human Mitochondrial Pathologies of the Respiratory Chain and ATP Synthase: Contributions from Studies of Saccharomyces Cerevisiae
life Review Human Mitochondrial Pathologies of the Respiratory Chain and ATP Synthase: Contributions from Studies of Saccharomyces cerevisiae Leticia V. R. Franco 1,2,* , Luca Bremner 1 and Mario H. Barros 2 1 Department of Biological Sciences, Columbia University, New York, NY 10027, USA; [email protected] 2 Department of Microbiology,Institute of Biomedical Sciences, Universidade de Sao Paulo, Sao Paulo 05508-900, Brazil; [email protected] * Correspondence: [email protected] Received: 27 October 2020; Accepted: 19 November 2020; Published: 23 November 2020 Abstract: The ease with which the unicellular yeast Saccharomyces cerevisiae can be manipulated genetically and biochemically has established this organism as a good model for the study of human mitochondrial diseases. The combined use of biochemical and molecular genetic tools has been instrumental in elucidating the functions of numerous yeast nuclear gene products with human homologs that affect a large number of metabolic and biological processes, including those housed in mitochondria. These include structural and catalytic subunits of enzymes and protein factors that impinge on the biogenesis of the respiratory chain. This article will review what is currently known about the genetics and clinical phenotypes of mitochondrial diseases of the respiratory chain and ATP synthase, with special emphasis on the contribution of information gained from pet mutants with mutations in nuclear genes that impair mitochondrial respiration. Our intent is to provide the yeast mitochondrial specialist with basic knowledge of human mitochondrial pathologies and the human specialist with information on how genes that directly and indirectly affect respiration were identified and characterized in yeast. Keywords: mitochondrial diseases; respiratory chain; yeast; Saccharomyces cerevisiae; pet mutants 1. -

Genome-Wide Investigation of Cellular Functions for Trna Nucleus
Genome-wide Investigation of Cellular Functions for tRNA Nucleus- Cytoplasm Trafficking in the Yeast Saccharomyces cerevisiae DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Hui-Yi Chu Graduate Program in Molecular, Cellular and Developmental Biology The Ohio State University 2012 Dissertation Committee: Anita K. Hopper, Advisor Stephen Osmani Kurt Fredrick Jane Jackman Copyright by Hui-Yi Chu 2012 Abstract In eukaryotic cells tRNAs are transcribed in the nucleus and exported to the cytoplasm for their essential role in protein synthesis. This export event was thought to be unidirectional. Surprisingly, several lines of evidence showed that mature cytoplasmic tRNAs shuttle between nucleus and cytoplasm and their distribution is nutrient-dependent. This newly discovered tRNA retrograde process is conserved from yeast to vertebrates. Although how exactly the tRNA nuclear-cytoplasmic trafficking is regulated is still under investigation, previous studies identified several transporters involved in tRNA subcellular dynamics. At least three members of the β-importin family function in tRNA nuclear-cytoplasmic intracellular movement: (1) Los1 functions in both the tRNA primary export and re-export processes; (2) Mtr10, directly or indirectly, is responsible for the constitutive retrograde import of cytoplasmic tRNA to the nucleus; (3) Msn5 functions solely in the re-export process. In this thesis I focus on the physiological role(s) of the tRNA nuclear retrograde pathway. One possibility is that nuclear accumulation of cytoplasmic tRNA serves to modulate translation of particular transcripts. To test this hypothesis, I compared expression profiles from non-translating mRNAs and polyribosome-bound translating mRNAs collected from msn5Δ and mtr10Δ mutants and wild-type cells, in fed or acute amino acid starvation conditions. -

Output Results of CLIME (Clustering by Inferred Models of Evolution)
Output results of CLIME (CLustering by Inferred Models of Evolution) Dataset: Num of genes in input gene set: 81 Total number of genes: 20834 Prediction LLR threshold: 0 The CLIME PDF output two sections: 1) Overview of Evolutionarily Conserved Modules (ECMs) Top panel shows the predefined species tree. Bottom panel shows the partition of input genes into Evolutionary Conserved Modules (ECMs), ordered by ECM strength (shown at right), and separated by horizontal lines. Each row show one gene, where the phylogenetic profile indicates presence (blue) or absence (gray) of homologs in each species (column). Gene symbols are shown at left. Gray color indicates that the gene is a paralog to a higher scoring gene within the same ECM (based on BLASTP E < 1e-3). 2) Details of each ECM and its expansion ECM+ Top panel shows the inferred evolutionary history on the predefined species tree. Branch color shows the gain event (blue) and loss events (red color, with brighter color indicating higher confidence in loss). Branches before the gain or after a loss are shown in gray. Bottom panel shows the input genes that are within the ECM (blue/white rows) as well as all genes in the expanded ECM+ (green/gray rows). The ECM+ includes genes likely to have arisen under the inferred model of evolution relative to a background model, and scored using a log likelihood ratio (LLR). PG indicates "paralog group" and are labeled alphabetically (i.e., A, B). The first gene within each paralog group is shown in black color. All other genes sharing sequence similarity (BLAST E < 1e-3) are assigned to the same PG label and displayed in gray. -

Novel Pathogenic COX20 Variants Causing Dysarthria, Ataxia, and Sensory Neuropathy
UCLA UCLA Previously Published Works Title Novel pathogenic COX20 variants causing dysarthria, ataxia, and sensory neuropathy. Permalink https://escholarship.org/uc/item/3s51m3n7 Journal Annals of clinical and translational neurology, 6(1) ISSN 2328-9503 Authors Otero, Maria G Tiongson, Emmanuelle Diaz, Frank et al. Publication Date 2019 DOI 10.1002/acn3.661 Peer reviewed eScholarship.org Powered by the California Digital Library University of California BRIEF COMMUNICATION Novel pathogenic COX20 variants causing dysarthria, ataxia, and sensory neuropathy Maria G. Otero1,a, Emmanuelle Tiongson2,a, Frank Diaz3, Katrina Haude4, Karin Panzer5, Ashley Collier6, Jaemin Kim1, David Adams7,8, Cynthia J. Tifft7,8, Hong Cui4, Francisca Millian Zamora4, Margaret G. Au9, John M. Graham Jr9, David J. Buckley10, Richard Lewis3, Camilo Toro7,8, Renkui Bai4, Lesley Turner11, Katherine D. Mathews6,12, William Gahl7,8 & Tyler Mark Pierson1,3,9 1Board of Governors Regenerative Medicine Institute, Cedars-Sinai Medical Center, Los Angeles, California 2Division of Neurology, Children’s Hospital of Los Angeles, Los Angeles, California 3Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, California 4GeneDx, Gaithersburg, Maryland 5Department of Pediatrics, University of Iowa Stead Family Children’s Hospital, Iowa City, Iowa 6Provincial Medical Genetics Program, Eastern Health, St. John’s, Newfoundland and Labrador, Canada 7NIH Undiagnosed Diseases Program, NIH Office of Rare Diseases Research and NHGRI, Bethesda, Maryland 8Office of the Clinical Director, NHGRI, NIH, Bethesda, Maryland 9Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, California 10Department of Pediatrics, Janeway Health Centre, St. John’s, Newfoundland and Labrador, Canada 11Faculty of Medicine, Memorial University of Newfoundland, St. John’s, Newfoundland, Canada 12Department of Neurology, University of Iowa Stead Family Children’s Hospital, Iowa City, Iowa Correspondence Abstract Tyler M.