Lecture 10: Mantle Minerals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineralogy and Geochemistry of Nephrite Jade from Yinggelike Deposit, Altyn Tagh (Xinjiang, NW China)
minerals Article Mineralogy and Geochemistry of Nephrite Jade from Yinggelike Deposit, Altyn Tagh (Xinjiang, NW China) Ying Jiang 1, Guanghai Shi 1,* , Liguo Xu 2 and Xinling Li 3 1 State Key Laboratory of Geological Processes and Mineral Resources, China University of Geosciences, Beijing 100083, China; [email protected] 2 Geological Museum of China, Beijing 100034, China; [email protected] 3 Xinjiang Uygur Autonomous Region Product Quality Supervision and Inspection Institute, Xinjiang 830004, China; [email protected] * Correspondence: [email protected]; Tel.: +86-010-8232-1836 Received: 6 April 2020; Accepted: 6 May 2020; Published: 8 May 2020 Abstract: The historic Yinggelike nephrite jade deposit in the Altyn Tagh Mountains (Xinjiang, NW China) is renowned for its gem-quality nephrite with its characteristic light-yellow to greenish-yellow hue. Despite the extraordinary gemological quality and commercial significance of the Yinggelike nephrite, little work has been done on this nephrite deposit, due to its geographic remoteness and inaccessibility. This contribution presents the first systematic mineralogical and geochemical studies on the Yinggelike nephrite deposit. Electron probe microanalysis, X-ray fluorescence (XRF) spectrometry, inductively coupled plasma mass spectrometry (ICP-MS) and isotope ratio mass spectrometry were used to measure the mineralogy, bulk-rock chemistry and stable (O and H) isotopes characteristics of samples from Yinggelike. Field investigation shows that the Yinggelike nephrite orebody occurs in the dolomitic marble near the intruding granitoids. Petrographic studies and EMPA data indicate that the nephrite is mainly composed of fine-grained tremolite, with accessory pargasite, diopside, epidote, allanite, prehnite, andesine, titanite, zircon, and calcite. Geochemical studies show that all nephrite samples have low bulk-rock Fe/(Fe + Mg) values (0.02–0.05), as well as low Cr (0.81–34.68 ppm), Co (1.10–2.91 ppm), and Ni (0.52–20.15 ppm) contents. -

Perovskites: Crystal Structure, Important Compounds and Properties
Perovskites: crystal structure, important compounds and properties Peng Gao GMF Group Meeting 12,04,2016 Solar energy resource PV instillations Global Power Demand Terrestrial sun light To start • We have to solve the energy problem. • Any technology that has good potential to cut carbon emissions by > 10 % needs to be explored aggressively. • Researchers should not be deterred by the struggles some companies are having. • Someone needs to invest in scaling up promising solar cell technologies. Origin And History of Perovskite compounds Perovskite is calcium titanium oxide or calcium titanate, with the chemical formula CaTiO3. The mineral was discovered by Gustav Rose in 1839 and is named after Russian mineralogist Count Lev Alekseevich Perovski (1792–1856).” All materials with the same crystal structure as CaTiO3, namely ABX3, are termed perovskites: Origin And History of Perovskite compounds Very stable structure, large number of compounds, variety of properties, many practical applications. Key role of the BO6 octahedra in ferromagnetism and ferroelectricity. Extensive formation of solid solutions material optimization by composition control and phase transition engineering. A2+ B4+ O2- Ideal cubic perovskite structure (ABO3) Classification of Perovskite System Perovskite Systems Inorganic Halide Oxide Perovskites Perovskites Alkali-halide Organo-Metal Intrinsic Doped Perovskites Halide Perovskites Perovskites A2Cl(LaNb2)O7 Perovskites 1892: 1st paper on lead halide perovskites Structure deduced 1959: Kongelige Danske Videnskabernes -

Descriptive Mineralogy
Descriptive Mineralogy Oxides and Hydroxides Classification of the Minerals • Non-Silicates • Silicates – Native Elements – Orthosilicates – Halides – Sorosilicates – Sulfides – Cyclosilicates – Oxides – Chain Silicates – Hydroxides – Layer Silicates – Carbonates – Tektosilicates – Sulfates – Phosphates Simple Oxides • Hemioxides • Sesquioxides – Cuprite (Cu O) 2 – Corundum (Al2O3) – Ice (H O) 2 – Hematite (Fe2O3) • Monoxides – Bixbyite (Mn2O3) – Periclase (MgO) • Dioxides – Wüstite (FeO) – Rutile (TiO2) – Manganosite (MnO) – Anatase (TiO2) – Lime (CaO) – Brookite (TiO2) – Zincite (ZnO) – Cassiterite(SnO2) – Bromellite (BeO) – Pyrolusite(MnO2) – Tenorite (CuO) Simple Oxides Hemi-Oxides (M2O) • Ice (H2O) Hexagonal • Cuprite (Cu2O) • Why not Na2O? – (Na radius too large) Cuprite Cu2O • Occurrence: Low Temp Hydrothermal (Supergene) • Use: Minor ore of Cu Ice H2O Crystal System Hexagonal Point Group 6/mmm Space Group P63/mmc Optical Uniaxial Color Colorless Luster Vitreous Hardness 1.5 Density 0.95 Ice H2O Ice H2O Ice H2O High Pressure Phase Diagram Monoxides (MO) • Rocksalt oxides – Periclase MgO - Wüstite FeO – Manganosite MnO – Lime CaO – Bunsenite NiO • Zincite oxides: – Zincite ZnO, – Bromellite BeO • Other monoxides: – TenoriteCuO, Montroydite HgO Rocksalt Oxides MgO-FeO-MnO-CaO Crystal System Cubic Point Group 4/m-32/m Space Group Fm3m Optical Isotropic Periclase - Wüstite MgO - FeO Lower mantle phase Mg2SiO4 = MgSiO3 + MgO Ringwoodite = Perovskite + periclase Periclase MgO Sesquioxides (M2O3) • Corundum Group – Corundum Al2O3 – Hematite -

Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
975 The Canadian Mineralogist Vol. 38, pp. 975-994 (2000) OCCURRENCE, ALTERATION PATTERNS AND COMPOSITIONAL VARIATION OF PEROVSKITE IN KIMBERLITES ANTON R. CHAKHMOURADIAN§ AND ROGER H. MITCHELL Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT The present work summarizes a detailed investigation of perovskite from a representative collection of kimberlites, including samples from over forty localities worldwide. The most common modes of occurrence of perovskite in archetypal kimberlites are discrete crystals set in a serpentine–calcite mesostasis, and reaction-induced rims on earlier-crystallized oxide minerals (typically ferroan geikielite or magnesian ilmenite). Perovskite precipitates later than macrocrystal spinel (aluminous magnesian chromite), and nearly simultaneously with “reaction” Fe-rich spinel (sensu stricto), and groundmass spinels belonging to the magnesian ulvöspinel – magnetite series. In most cases, perovskite crystallization ceases prior to the resorption of groundmass spinels and formation of the atoll rim. During the final evolutionary stages, perovskite commonly becomes unstable and reacts with a CO2- rich fluid. Alteration of perovskite in kimberlites involves resorption, cation leaching and replacement by late-stage minerals, typically TiO2, ilmenite, titanite and calcite. Replacement reactions are believed to take place at temperatures below 350°C, 2+ P < 2 kbar, and over a wide range of a(Mg ) values. Perovskite from kimberlites approaches the ideal formula CaTiO3, and normally contains less than 7 mol.% of other end-members, primarily lueshite (NaNbO3), loparite (Na0.5Ce0.5TiO3), and CeFeO3. Evolutionary trends exhibited by perovskite from most localities are relatively insignificant and typically involve a decrease in REE and Th contents toward the rim (normal pattern of zonation). -

In Situ X-Ray Diffraction Study of Phase Transitions of Fetio3 at High Pressures and Temperatures Using a Large-Volume Press and Synchrotron Radiation
American Mineralogist, Volume 91, pages 120–126, 2006 In situ X-ray diffraction study of phase transitions of FeTiO3 at high pressures and temperatures using a large-volume press and synchrotron radiation LI CHUNG MING,1,* YOUNG-HO KIM,2 T. UCHIDA,3 Y. WANG,3 AND M. RIVERS3 1Hawaii Institute of Geophysics and Planetology, University of Hawaii, Honolulu, Hawaii 96822, U.S.A. 2Department of Earth and Environment Science, Gyeongsang National University, Jinju 660-701, Korea 3The University of Chicago, 5640 South Ellis Avenue, Chicago, Illinois 60637, U.S.A. ABSTRACT The phase transformation from ilmenite to perovskite in FeTiO3 was directly observed using synchrotron-based X-ray diffraction and a large-volume press. The perovskite phase is temperature quenchable at 20 GPa and converts into the LiNbO3 phase at pressures below 15 GPa at room tem- perature. The LiNbO3 phase transforms into the ilmenite phase at 10 GPa and 673 K. However, the back-transformation from the ilmenite to the LiNbO3 phase was not observed, thus strongly suggesting that the LiNbO3 phase is not thermodynamically stable but rather a retrogressive phase formed from perovskite during decompression at room temperature. By cycling the pressure up and down at temperatures between 773 and 1023 K, the perovskite- ilmenite transformation could be observed in both directions, thus conÞ rming that perovskite is the true high-pressure phase with respect to the ilmenite phase at lower pressures. The phase boundary of the perovskite-ilmenite transformation thus determined in this study is represented by P (GPa) = 16.0 (±1.4) – 0.0012 (±0.0014) T (K), which is inconsistent with P = 25.2 – 0.01 T (K) reported previously (Syono et al. -
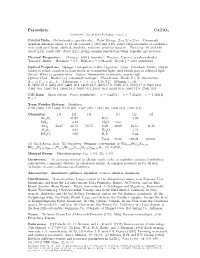
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique
Hindawi Advances in Materials Science and Engineering Volume 2019, Article ID 1612456, 7 pages https://doi.org/10.1155/2019/1612456 Research Article Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique Oratai Jongprateep ,1,2 Nicha Sato ,1 Ratchatee Techapiesancharoenkij,1,2 and Krissada Surawathanawises1 1Department of Materials Engineering, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand 2Materials Innovation Center, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand Correspondence should be addressed to Oratai Jongprateep; [email protected] Received 29 October 2018; Accepted 13 February 2019; Published 4 April 2019 Academic Editor: Alexander Kromka Copyright © 2019 Oratai Jongprateep et al. *is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Calcium titanate (CaTiO3), strontium titanate (SrTiO3), and strontium calcium titanate (SrxCa1−xTiO3) are widely recognized and utilized as dielectric materials. *eir electrocatalytic properties, however, have not been extensively examined. *e aim of this research is to explore the electrocatalytic performance of calcium titanate, strontium titanate, and strontium calcium titanate, as potential sensing materials. Experimental results revealed that CaTiO3, SrTiO3, and Sr0.5Ca0.5TiO3 powders synthesized by the solution combustion technique consisted of submicrometer-sized particles with 2 specific surface areas ranging from 4.19 to 5.98 m /g. Optical bandgap results indicated that while CaTiO3 and SrTiO3 had bandgap energies close to 3 eV, Sr0.5Ca0.5TiO3 yielded a lower bandgap energy of 2.6 eV. Cyclic voltammetry tests, measured in 0.1 M sodium nitrite, showed oxidation peaks occurring at 0.58 V applied voltage. -

TEM-HRTEM Study on the Dehydration Process of Nanostructured Mg-Ca 3 4 5 Hydroxide Into Mg-Ca Oxide 6 7 8 L.S
*Manuscript Click here to view linked References 1 2 TEM-HRTEM study on the dehydration process of nanostructured Mg-Ca 3 4 5 hydroxide into Mg-Ca oxide 6 7 8 L.S. Gomez-Villalba1*, A. Sierra-Fernandez1,2 , M.E. Rabanal2,3 R.Fort1 9 10 11 1 Instituto de Geociencias (CSIC, UCM), Calle José Antonio Novais 12, 28040 Madrid, Spain 12 13 14 2 Materials Science and Engineering Department Universidad Carlos III de Madrid, Avda. 15 16 17 Universidad 30, 28911 Leganés, Madrid, Spain 18 19 20 3 Instituto Tecnológico de Química y Materiales Alvaro Alonso Barba (IAAB) , Avda. 21 22 Universidad 30, 28911 Leganés, Madrid, Spain 23 24 25 26 KEYWORDS: Nanoparticles, crystal growth, defects, HRTEM, EELS, SAED, radiolysis. Brucite, 27 28 periclase 29 30 31 * Corresponding author: Luz Stella Gomez-Villalba [email protected], 32 33 34 [email protected] 35 36 37 38 Abstract 39 40 41 The dehydration process from Mg 0.97Ca0.03 (OH)2 nanoparticles (brucite type hexagonal 42 43 44 structure) to Mg0.97Ca0.03 O (periclase type cubic structure) was studied by Transmission 45 46 Electron Microscopy (TEM-HRTEM), Electron Diffraction (SAED), EELS spectroscopy (EELS) and 47 48 image analysis. The transformation process was monitored in function of the reaction time 49 50 51 applying 200 and 300 KV. Changes in porosity were possible to observe only during the 52 53 irradiation with 200 KV. Depending on the irradiation time, the changes were gradual, 54 55 producing an increase from the particle´s edge towards the inner region. Different stages were 56 57 58 observed, corresponding to the amount of water extracted from the particle, until finally a 59 60 decrease in porosity and particle shrinkage occurs, coinciding with the formation of the Mg-Ca 61 62 1 63 64 65 oxide. -

Quantification of Water in Hydrous Ringwoodite
ORIGINAL RESEARCH ARTICLE published: 28 January 2015 EARTH SCIENCE doi: 10.3389/feart.2014.00038 Quantification of water in hydrous ringwoodite Sylvia-Monique Thomas 1*,StevenD.Jacobsen2, Craig R. Bina 2, Patrick Reichart 3, Marcus Moser 3, Erik H. Hauri 4, Monika Koch-Müller 5,JosephR.Smyth6 and Günther Dollinger 3 1 Department of Geoscience, University of Nevada Las Vegas, Las Vegas, NV, USA 2 Department of Earth and Planetary Sciences, Northwestern University, Evanston, IL, USA 3 Department für Luft- und Raumfahrttechnik LRT2, Universität der Bundeswehr München, Neubiberg, Germany 4 Department of Terrestrial Magnetism, Carnegie Institution of Washington, Washington, DC, USA 5 Helmholtz-Zentrum Potsdam, Deutsches GeoForschungsZentrum (GFZ), Potsdam, Germany 6 Department of Geological Sciences, University of Colorado Boulder, Boulder, CO, USA Edited by: Ringwoodite, γ-(Mg,Fe)2SiO4, in the lower 150 km of Earth’s mantle transition zone Mainak Mookherjee, Cornell (410–660 km depth) can incorporate up to 1.5–2wt% H2O as hydroxyl defects. We University, USA present a mineral-specific IR calibration for the absolute water content in hydrous Reviewed by: ringwoodite by combining results from Raman spectroscopy, secondary ion mass Geoffrey David Bromiley, University of Edinburgh, UK spectrometry (SIMS) and proton-proton (pp)-scattering on a suite of synthetic Mg- and Marc Blanchard, CNRS - Université Fe-bearing hydrous ringwoodites. H2O concentrations in the crystals studied here Pierre et Marie Curie, France range from 0.46 to 1.7wt% H2O (absolute methods), with the maximum H2Oin *Correspondence: the same sample giving 2.5 wt% by SIMS calibration. Anchoring our spectroscopic Sylvia-Monique Thomas, results to absolute H-atom concentrations from pp-scattering measurements, we report Department of Geoscience, University of Nevada Las Vegas, frequency-dependent integrated IR-absorption coefficients for water in ringwoodite −1 −2 4505 S. -

The American Mineralogist Journal of the Mineralogical Society of America
THE AMERICAN MINERALOGIST JOURNAL OF THE MINERALOGICAL SOCIETY OF AMERICA Vol. 46 MAY-JUNE, 1961 Nos. 5 and 6 KIMZEYITE, A ZIRCONIUM GARNET FROM MAGNET COVE, ARKANSAS* CHARLES MILTON, BLANCHE L. INGRAM, AND LAWRENCE V. BLADE, U. S. Geological Survey, Washington, D. C. ABSTRACT Kimzeyite, Ca3(Zr, 'Ii, Mg, Fe", Nb)z(AI, Fe"', Si),012, is a new type of garnet oc- curring as dodecahedrons modified by trapezohedron at Magnet Cove, Arkansas, in a carbonatite with abundant apatite, monticellite, calcite, perovskite (dysanalyte), magne- tite, and minor biotite, pyrite, and vesuvianite. It is dark brown, H about 7, isotropic, in- soluble in acids, infusible before the blowpipe, D = 4.0, n = 1.94. The three strongest x-ray powder pattern lines with intensities as measured are 1.667 (10), 2.539 (9), 2.79 (8); the unit cell constant is 12.46 A. Microchemical analysis gave CaO 29.8, Zr02 29.9, Ti203 5.0, MgO 0.5, FeO 0.8, Nb205 1.0, AhO" 11.0, Fe203 13.4, Si02 9.6, sum 101.0 which computes to Cas.n (Zrl.42+4Tio.4o+3Mgo.o7+21'eO.07+2Nbu. 05+;)(Ah.2. +3Feo.98+3Sio. 94+4)012.00. Basically, this is Ca"Zr2(AI,Si)012 with Ti replacing Zr and Fe replacing AI. Zirconium has been found in other garnets (schor]omite from Magnet Cove, melanite from Kaiserstuhl, Germany, and titanian andradite from Oka, Quebec) in quantity up to severa] per cent, but in none of these is it a major constituent. Kimzeyite is named in honor of the Kimzey family, long known in connection with Magnet Cove mineralogy. -

Lower Mantle Hydrogen Partitioning Between Periclase and Perovskite: a Quantum Chemical Modelling
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archivio istituzionale della ricerca - Università di Palermo Available online at www.sciencedirect.com ScienceDirect Geochimica et Cosmochimica Acta 173 (2016) 304–318 www.elsevier.com/locate/gca Lower mantle hydrogen partitioning between periclase and perovskite: A quantum chemical modelling Marcello Merli a, Costanza Bonadiman b, Valeria Diella c, Alessandro Pavese c,d,⇑ a Department of Earth and Marine Sciences, University of Palermo, Via Archirafi 36, 90123 Palermo, Italy b Department of Physics and Earth Sciences, University of Ferrara, Via Saragat 1, 44122 Ferrara, Italy c Consiglio Nazionale delle Ricerche, CNR-IDPA, Section of Milan, Via Mangiagalli 34, 20133 Milan, Italy d Department of Earth Sciences ‘‘A. Desio”, University of Milan, Via Botticelli 23, 20133 Milan, Italy Received 2 April 2015; accepted in revised form 27 October 2015; available online 6 November 2015 Abstract Partitioning of hydrogen (often referred to as H2O) between periclase (pe) and perovskite (pvk) at lower mantle conditions (24–80 GPa) was investigated using quantum mechanics, equilibrium reaction thermodynamics and by monitoring two H-incorporation models. One of these (MSWV) was based on replacements provided by Mg2+ M 2H+ and Si4+ M 4H+; while 2+ 3+ + 4+ 3+ + the other (MSWA) relied upon substitutions in 2Mg M Al +H and Si M Al +H .H2O partitioning in these phases was considered in the light of homogeneous (Bulk Silicate Earth; pvk: 75%–pe:16% model contents) and heterogeneous (Layered Mantle; pvk:78%–pe:14% modal contents) mantle geochemical models, which were configured for lower and upper bulk water contents (BWC) at 800 and 1500 ppm, respectively. -

Seismic Evidence for Water Deep in Earth's Upper Mantle
R EPORTS million years ago (Ma), subduction mainly took place in the eastern Mediterranean with Seismic Evidence for Water Deep mostly south- and eastward-dipping subduc- tion. Remnants of the subducted slab can be in Earth’s Upper Mantle found in the lower mantle between 1300 and 1900 km depth under locations such as Egypt Mark van der Meijde,* Federica Marone, Domenico Giardini, and Libya (18). Subduction was ubiquitous Suzan van der Lee after 80 Ma. At present, active subduction zones are found in southern Italy and Greece. Water in the deep upper mantle can influence the properties of seismic dis- We investigated seismic discontinuities in the continuities in the mantle transition zone. Observations of converted seismic deep upper mantle of the Mediterranean re- waves provide evidence of a 20- to 35-kilometer-thick discontinuity near a gion by searching seismograms for seismic S depth of 410 kilometers, most likely explained by as much as 700 parts per waves that converted from P waves at the million of water by weight. discontinuities. Our search was facilitated by a recent temporary deployment of mobile Two major seismic velocity discontinuities, lies in the mantle transition zone would broadband seismic stations [MIDSEA (19)] at nominal depths of 410 and 660 km, border have no seismically observable effect on along the plate boundary between Eurasia the transition zone of Earth’s mantle. The the thickness of the 660-km discontinuity and Africa. We analyzed over 500 seismo- 410-km discontinuity is the result of the tran- (7), whereas the effect on the 520-km dis- grams recorded in the Mediterranean region sition from olivine to wadsleyite.