Knockdown of EIF5A2 Inhibits the Malignant Potential of Non-Small Cell Lung Cancer Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Translational Control in Cancer Etiology
Downloaded from http://cshperspectives.cshlp.org/ on October 1, 2021 - Published by Cold Spring Harbor Laboratory Press Translational Control in Cancer Etiology Davide Ruggero Helen Diller Cancer Center, School of Medicine, University of California, San Francisco, California 94158 Correspondence: [email protected] The link between perturbations in translational control and cancer etiology is becoming a primary focus in cancer research. It has now been established that genetic alterations in several components of the translational apparatus underlie spontaneous cancers as well as an entire class of inherited syndromes known as “ribosomopathies” associated with in- creased cancer susceptibility. These discoveries have illuminated the importance of dereg- ulations in translational control to very specific cellular processes that contribute to cancer etiology. In addition, a growing body of evidence supports the view that deregulation of translational control is a common mechanism by which diverse oncogenic pathways promote cellular transformation and tumor development. Indeed, activation of these key oncogenic pathways induces rapid and dramatic translational reprogramming both by in- creasing overall protein synthesis and by modulating specific mRNA networks. These trans- lational changes promote cellular transformation, impacting almost every phase of tumor development. This paradigm represents a new frontier in the multihit model of cancer for- mation and offers significant promise for innovative cancer therapies. Current research, -
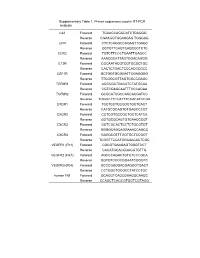
Supplementary Table 1. Primer Sequences Used in RT-PCR Analysis
Supplementary Table 1. Primer sequences used in RT-PCR analysis L32 Forward TGAAGCAGGCATCTGAGGG Reverse CGAAGGTGGAAGAG TGGGAG LIFR Forward CTCTCAGGCCAGAGTTGAGC Reverse GCTGTTCAGTCAGCCCTCTC CCR2 Forward TGTCTTCCCTGAATTGAGCC Reverse AAACGCATTAGTGGACAGGG IL12R Forward CGCAATACGTCGTGCGCTGC Reverse CACTCTGACTCCCACGCGCC CSF1R Forward GCTGGTGCGGATTCGAGGGG Reverse TTCGGCGTTAGTGGCCGAGC TGFbR1 Forward ACGCGCTGACATCTATGCAA Reverse CGTCGAGCAATTTCCCAGAA TGFbR2 Forward GCGCATCGCCAGCACGATCC Reverse TGGGCTTCCATTTCCACATCCGA CXCR1 Forward TCCTCCTGCCGCTGCTCACT Reverse CATGCGCAGTGTGAGCCCGT CXCR2 Forward CCTCGTGCCGCTGCTCATCA Reverse GGTGCGCAGTGTGAACCCGT CXCR3 Forward GGTCGCACTGCTCTGCGTGT Reverse GGGGCAGCAGGAAACCAGCC CXCR4 Forward GAGGCGTTTGGTGCTCCGGT Reverse TCGGTTCCATGGCAACACTCGC VEGFR1 (Flt1) Forward CGCGTGAAGAGTGGGTCCT Reverse CACATGCACGGAGGTGTTG VEGFR2 (Flk1) Forward AGCCCAGACTGTGTCCCGCA Reverse GGTGTCCGCGGAATCGGGTC VEGFR3 (Flt4) Forward GCCCGAGGACGAGGGTGACT Reverse CCTGGCTGCGCCTATCCTGC human Flt1 Forward GCACGTCAGCGAAGGCAAGC Reverse CCAGCTCAGCGTGGTCGTAGG Supplementary Table 2. List of putative miR-200 target genes amplified in human cancers amplified in cancers (Tumorscape)a Gene Symbol Title various cancers NSCLC PCT Errfi1 ERBB receptor feedback inhibitor 1 - yes 0.94 D11Bwg0517e DNA segment, Chr 11, Brigham & Women's Genetics 0517 expressed - yes 0.91 Pvrl4 poliovirus receptor-related 4 yes - 0.91 Mecp2 methyl CpG binding protein 2 yes yes 0.9 Jun Jun oncogene yes - 0.88 Prdm16 PR domain containing 16 - yes 0.88 Flt1 FMS-like tyrosine kinase 1 yes -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

EIF5A2 Controls Ovarian Tumor Growth and Metastasis by Promoting Epithelial to Mesenchymal Transition Via the Title Tgfβ Pathway
EIF5A2 controls ovarian tumor growth and metastasis by promoting epithelial to mesenchymal transition via the Title TGFβ pathway Zhao, Guannan; Zhang, Wenjing; Dong, Peixin; Watari, Hidemichi; Guo, Yuqi; Pfeffer, Lawrence M; Tigyi, Gabor; Author(s) Yue, Junming Cell & Bioscience, 11, 70 Citation https://doi.org/10.1186/s13578-021-00578-5 Issue Date 2021-04-07 Doc URL http://hdl.handle.net/2115/80967 Rights(URL) https://creativecommons.org/licenses/by/4.0/ Type article File Information Yue EIF5A2 paper.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Zhao et al. Cell Biosci (2021) 11:70 https://doi.org/10.1186/s13578-021-00578-5 Cell & Bioscience RESEARCH Open Access EIF5A2 controls ovarian tumor growth and metastasis by promoting epithelial to mesenchymal transition via the TGFβ pathway Guannan Zhao1,2, Wenjing Zhang3, Peixin Dong4, Hidemichi Watari4, Yuqi Guo5*, Lawrence M. Pfefer1,2, Gabor Tigyi6 and Junming Yue1,2* Abstract Background: Epithelial to mesenchymal transition (EMT) contributes to tumor metastasis and chemoresistance. Eukaryotic initiation factor 5A2 (EIF5A2) is highly expressed in a variety of human cancers but rarely expressed in normal tissues. While EIF5A2 has oncogenic activity in several cancers and contributes to tumor metastasis, its role in ovarian cancer is unknown. In this study, we investigate whether EIF5A2 contributes to ovarian tumor metastasis by promoting EMT. Methods: To investigate the role of EIF5A2, we knocked out (KO) EIF5A2 using lentiviral CRISPR/Cas9 nickase in high invasive SKOV3 and OVCAR8 cells and overexpressed EIF5A2 in low invasive OVCAR3 cells using lentiviral vector. Cell proliferation, migration and invasion was examined in vitro ovarian cancer cells and tumor metastasis was evaluated in vivo using orthotopic ovarian cancer mouse models. -

WO 2019/079361 Al 25 April 2019 (25.04.2019) W 1P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2019/079361 Al 25 April 2019 (25.04.2019) W 1P O PCT (51) International Patent Classification: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, C12Q 1/68 (2018.01) A61P 31/18 (2006.01) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, C12Q 1/70 (2006.01) HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, (21) International Application Number: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, PCT/US2018/056167 OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (22) International Filing Date: SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 16 October 2018 (16. 10.2018) TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (26) Publication Language: English GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, (30) Priority Data: UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, 62/573,025 16 October 2017 (16. 10.2017) US TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, ΓΕ , IS, IT, LT, LU, LV, (71) Applicant: MASSACHUSETTS INSTITUTE OF MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, TECHNOLOGY [US/US]; 77 Massachusetts Avenue, TR), OAPI (BF, BJ, CF, CG, CI, CM, GA, GN, GQ, GW, Cambridge, Massachusetts 02139 (US). -

Translational Control in Cancer Etiology
Downloaded from http://cshperspectives.cshlp.org/ on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press Translational Control in Cancer Etiology Davide Ruggero Helen Diller Cancer Center, School of Medicine, University of California, San Francisco, California 94158 Correspondence: [email protected] The link between perturbations in translational control and cancer etiology is becoming a primary focus in cancer research. It has now been established that genetic alterations in several components of the translational apparatus underlie spontaneous cancers as well as an entire class of inherited syndromes known as “ribosomopathies” associated with in- creased cancer susceptibility. These discoveries have illuminated the importance of dereg- ulations in translational control to very specific cellular processes that contribute to cancer etiology. In addition, a growing body of evidence supports the view that deregulation of translational control is a common mechanism by which diverse oncogenic pathways promote cellular transformation and tumor development. Indeed, activation of these key oncogenic pathways induces rapid and dramatic translational reprogramming both by in- creasing overall protein synthesis and by modulating specific mRNA networks. These trans- lational changes promote cellular transformation, impacting almost every phase of tumor development. This paradigm represents a new frontier in the multihit model of cancer for- mation and offers significant promise for innovative cancer therapies. Current research, -

Proteomics of Stress Responses in Wheat and Barley—Search for Potential Protein Markers of Stress Tolerance
MINI REVIEW ARTICLE published: 11 December 2014 doi: 10.3389/fpls.2014.00711 Proteomics of stress responses in wheat and barley—search for potential protein markers of stress tolerance Klára Kosová*, Pavel Vítámvás and Ilja T. Prášil Laboratory of Plant Stress Biology and Biotechnology, Division of Crop Genetics and Breeding, Department of Plant Genetics, Breeding and Product Quality, Crop Research Institute, Prague, Czech Republic Edited by: Wheat (Triticum aestivum; T. durum) and barley (Hordeum vulgare) agricultural production Jesus V. Jorrin Novo, University of is severely limited by various abiotic and biotic stress factors. Proteins are directly Cordoba, Spain involved in plant stress response so it is important to study proteome changes Reviewed by: under various stress conditions. Generally, both abiotic and biotic stress factors induce Martin Hajduch, Slovak Academy of Sciences, Slovakia profound alterations in protein network covering signaling, energy metabolism (glycolysis, Jesus V. Jorrin Novo, University of Krebs cycle, ATP biosynthesis, photosynthesis), storage proteins, protein metabolism, Cordoba, Spain several other biosynthetic pathways (e.g., S-adenosylmethionine metabolism, lignin Arkadiusz Kosmala, Institute of Plant metabolism), transport proteins, proteins involved in protein folding and chaperone Genetics of the Polish Academy of Sciences, Poland activities, other protective proteins (LEA, PR proteins), ROS scavenging enzymes as *Correspondence: well as proteins affecting regulation of plant growth and development. Proteins which Klára Kosová, Laboratory of Plant have been reported to reveal significant differences in their relative abundance or Stress Biology and Biotechnology, posttranslational modifications between wheat, barley or related species genotypes under Division of Crop Genetics and stress conditions are listed and their potential role in underlying the differential stress Breeding, Crop Research Institute, Drnovská 507, Prague 6 – Ruzyne,ˇ response is discussed. -

A Master Autoantigen-Ome Links Alternative Splicing, Female Predilection, and COVID-19 to Autoimmune Diseases
bioRxiv preprint doi: https://doi.org/10.1101/2021.07.30.454526; this version posted August 4, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. A Master Autoantigen-ome Links Alternative Splicing, Female Predilection, and COVID-19 to Autoimmune Diseases Julia Y. Wang1*, Michael W. Roehrl1, Victor B. Roehrl1, and Michael H. Roehrl2* 1 Curandis, New York, USA 2 Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, USA * Correspondence: [email protected] or [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.07.30.454526; this version posted August 4, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract Chronic and debilitating autoimmune sequelae pose a grave concern for the post-COVID-19 pandemic era. Based on our discovery that the glycosaminoglycan dermatan sulfate (DS) displays peculiar affinity to apoptotic cells and autoantigens (autoAgs) and that DS-autoAg complexes cooperatively stimulate autoreactive B1 cell responses, we compiled a database of 751 candidate autoAgs from six human cell types. At least 657 of these have been found to be affected by SARS-CoV-2 infection based on currently available multi-omic COVID data, and at least 400 are confirmed targets of autoantibodies in a wide array of autoimmune diseases and cancer. -

1 1 2 Pharmacological Dimerization and Activation of the Exchange
1 2 3 Pharmacological dimerization and activation of the exchange factor eIF2B antagonizes the 4 integrated stress response 5 6 7 *Carmela Sidrauski1,2, *Jordan C. Tsai1,2, Martin Kampmann2,3, Brian R. Hearn4, Punitha 8 Vedantham4, Priyadarshini Jaishankar4 , Masaaki Sokabe5, Aaron S. Mendez1,2, Billy W. 9 Newton6, Edward L. Tang6.7, Erik Verschueren6, Jeffrey R. Johnson6,7, Nevan J. Krogan6,7,, 10 Christopher S. Fraser5, Jonathan S. Weissman2,3, Adam R. Renslo4, and Peter Walter 1,2 11 12 1Department of Biochemistry and Biophysics, University of California, San Francisco, United 13 States 14 2Howard Hughes Medical Institute, University of California, San Francisco, United States 15 3Department of Cellular and Molecular Pharmacology, University of California, San Francisco, 16 United States 17 4Department of Pharmaceutical Chemistry and the Small Molecule Discovery Center, University 18 of California at San Francisco, United States 19 5Department of Molecular and Cellular Biology, College of Biological Sciences, University of 20 California, Davis, United States 21 6QB3, California Institute for Quantitative Biosciences, University of California, San Francisco, 22 United States 23 7Gladstone Institutes, San Francisco, United States 24 25 * Both authors contributed equally to this work 26 27 28 Abstract 29 30 The general translation initiation factor eIF2 is a major translational control point. Multiple 31 signaling pathways in the integrated stress response phosphorylate eIF2 serine-51, inhibiting 32 nucleotide exchange by eIF2B. ISRIB, a potent drug-like small molecule, renders cells 33 insensitive to eIF2α phosphorylation and enhances cognitive function in rodents by blocking 34 long-term depression. ISRIB was identified in a phenotypic cell-based screen, and its mechanism 35 of action remained unknown. -

Eukaryotic Initiation Factor 4A2 Promotes Experimental Metastasis
Chen et al. Journal of Experimental & Clinical Cancer Research (2019) 38:196 https://doi.org/10.1186/s13046-019-1178-z RESEARCH Open Access Eukaryotic initiation factor 4A2 promotes experimental metastasis and oxaliplatin resistance in colorectal cancer Zhan-Hong Chen1,2†, Jing-Jing Qi1†, Qi-Nian Wu3†, Jia-Huan Lu1†, Ze-Xian Liu1, Yun Wang1, Pei-Shan Hu1, Ting Li1, Jin-Fei Lin1, Xiang-Yuan Wu2, Lei Miao1, Zhao-Lei Zeng1, Dan Xie3, Huai-Qiang Ju1, Rui-Hua Xu1* and Feng Wang1* Abstract Background: Deregulation of protein translation control is a hallmark of cancers. Eukaryotic initiation factor 4A2 (EIF4A2) is required for mRNA binding to ribosome and plays an important role in translation initiation. However, little is known about its functions in colorectal cancer (CRC). Methods: Analysis of CRC transcriptome data from TCGA identified that EIF4A2 was associated with poor prognosis. Immunohistochemistry study of EIF4A2 was carried out in 297 paired colorectal tumor and adjacent normal tissue samples. In vitro and in vivo cell-biological assays were performed to study the biological functions of EIF4A2 on experimental metastasis and sensitivity to oxaliplatin treatment. Bioinformatic prediction, chromatin immunoprecipitation (ChIP) and dual-luciferase reporter assay were carried out to unveil the transcription factor of EIF4A2 regulation. Results: EIF4A2 Expression is significantly higher in colorectal tumors. Multivariate analysis suggests EIF4A2 as an independent predictor of overall, disease-free and progression-free survival. Dysfunction of EIF4A2 by genetic knock-down or small-molecule inhibitor silvestrol dramatically inhibited CRC invasion and migration, sphere formation and enhanced sensitivity to oxaliplatin treatment in vitro and in vivo. -

Supplementary Dataset S2
mitochondrial translational termination MRPL28 MRPS26 6 MRPS21 PTCD3 MTRF1L 4 MRPL50 MRPS18A MRPS17 2 MRPL20 MRPL52 0 MRPL17 MRPS33 MRPS15 −2 MRPL45 MRPL30 MRPS27 AURKAIP1 MRPL18 MRPL3 MRPS6 MRPS18B MRPL41 MRPS2 MRPL34 GADD45GIP1 ERAL1 MRPL37 MRPS10 MRPL42 MRPL19 MRPS35 MRPL9 MRPL24 MRPS5 MRPL44 MRPS23 MRPS25 ITB ITB ITB ITB ICa ICr ITL original ICr ICa ITL ICa ITL original ICr ITL ICr ICa mitochondrial translational elongation MRPL28 MRPS26 6 MRPS21 PTCD3 MRPS18A 4 MRPS17 MRPL20 2 MRPS15 MRPL45 MRPL52 0 MRPS33 MRPL30 −2 MRPS27 AURKAIP1 MRPS10 MRPL42 MRPL19 MRPL18 MRPL3 MRPS6 MRPL24 MRPS35 MRPL9 MRPS18B MRPL41 MRPS2 MRPL34 MRPS5 MRPL44 MRPS23 MRPS25 MRPL50 MRPL17 GADD45GIP1 ERAL1 MRPL37 ITB ITB ITB ITB ICa ICr original ICr ITL ICa ITL ICa ITL original ICr ITL ICr ICa translational termination MRPL28 MRPS26 6 MRPS21 PTCD3 C12orf65 4 MTRF1L MRPL50 MRPS18A 2 MRPS17 MRPL20 0 MRPL52 MRPL17 MRPS33 −2 MRPS15 MRPL45 MRPL30 MRPS27 AURKAIP1 MRPL18 MRPL3 MRPS6 MRPS18B MRPL41 MRPS2 MRPL34 GADD45GIP1 ERAL1 MRPL37 MRPS10 MRPL42 MRPL19 MRPS35 MRPL9 MRPL24 MRPS5 MRPL44 MRPS23 MRPS25 ITB ITB ITB ITB ICa ICr original ICr ITL ICa ITL ICa ITL original ICr ITL ICr ICa translational elongation DIO2 MRPS18B MRPL41 6 MRPS2 MRPL34 GADD45GIP1 4 ERAL1 MRPL37 2 MRPS10 MRPL42 MRPL19 0 MRPL30 MRPS27 AURKAIP1 −2 MRPL18 MRPL3 MRPS6 MRPS35 MRPL9 EEF2K MRPL50 MRPS5 MRPL44 MRPS23 MRPS25 MRPL24 MRPS33 MRPL52 EIF5A2 MRPL17 SECISBP2 MRPS15 MRPL45 MRPS18A MRPS17 MRPL20 MRPL28 MRPS26 MRPS21 PTCD3 ITB ITB ITB ITB ICa ICr ICr ITL original ITL ICa ICa ITL ICr ICr ICa original -

Eukaryotic Translation Initiation Factor 5A2 Is Highly Expressed in Prostate Cancer and Predicts Poor Prognosis
EXPERIMENTAL AND THERAPEUTIC MEDICINE 17: 3741-3747, 2019 Eukaryotic translation initiation factor 5A2 is highly expressed in prostate cancer and predicts poor prognosis JIAN LU1*, HONG-WEI ZHAO2*, YU CHEN3*, JIN-HUAN WEI3, ZHEN-HUA CHEN3, ZI-HAO FENG3, YONG HUANG3, WEI CHEN3, JUN-HANG LUO3 and YONG FANG3 1Department of Urology, Jiangmen Hospital, Sun Yat-Sen University, Jiangmen, Guangdong 529030; 2Department of Urology, Affiliated Yantai Yuhuangding Hospital, Qingdao University Medical College, Jinan, Shandong 264000; 3Department of Urology, The First Affiliated Hospital, Sun Yat‑sen University, Guangzhou, Guangdong 510080, P.R. China Received July 1, 2018; Accepted February 14, 2019 DOI: 10.3892/etm.2019.7331 Abstract. Eukaryotic translation initiation factor (EIF) 5A2 in prostate cancer and may be a potential predictor and thera- exerts important functions that regulate the development and peutic target in PCa patients. progression of cancers. The present study aimed to investi- gate the expression of EIF5A2 in prostate cancer (PCa) and Introduction its association with biological and prognostic significance. EIF5A2 mRNA and protein levels were analyzed in three Prostate cancer (PCa) is the leading cause of cancer-associated paired samples of freshly resected PCa and adjacent non-tumor mortality in men worldwide (1). Approximately 60,300 new tissues. Immunohistochemical staining was used to detect the PCa cases are diagnosed annually, and ~26,600 die of aggres- expression of EIF5A2 protein levels in 72 paraffin‑embedded sive PCa in China annually (2). Although many approaches PCa tumor specimens. Subsequently, the association between have been explored to improve the diagnosis and treatment of EIF5A2 protein expression and clinicopathological parameters PCa, the incidence and mortality of PCa has still increased was assessed.