Ribosome Profiling Reveals an Important Role for Translational Control in Circadian Gene Expression
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Translational Control in Cancer Etiology
Downloaded from http://cshperspectives.cshlp.org/ on October 1, 2021 - Published by Cold Spring Harbor Laboratory Press Translational Control in Cancer Etiology Davide Ruggero Helen Diller Cancer Center, School of Medicine, University of California, San Francisco, California 94158 Correspondence: [email protected] The link between perturbations in translational control and cancer etiology is becoming a primary focus in cancer research. It has now been established that genetic alterations in several components of the translational apparatus underlie spontaneous cancers as well as an entire class of inherited syndromes known as “ribosomopathies” associated with in- creased cancer susceptibility. These discoveries have illuminated the importance of dereg- ulations in translational control to very specific cellular processes that contribute to cancer etiology. In addition, a growing body of evidence supports the view that deregulation of translational control is a common mechanism by which diverse oncogenic pathways promote cellular transformation and tumor development. Indeed, activation of these key oncogenic pathways induces rapid and dramatic translational reprogramming both by in- creasing overall protein synthesis and by modulating specific mRNA networks. These trans- lational changes promote cellular transformation, impacting almost every phase of tumor development. This paradigm represents a new frontier in the multihit model of cancer for- mation and offers significant promise for innovative cancer therapies. Current research, -

Paul Hardin, Ph.D. John W
Department of Biology The College of Arts + Sciences | Indiana University Bloomington About Paul Hardin Distinguished Alumni Award Lecture Thu., Oct. 18, 2018 • 4 to 5 pm • Myers Hall 130 Paul Hardin, Ph.D. John W. Lyons Jr. ’59 Chair in Biology, Texas A&M University Genetic architecture underlying circadian clock initiation, maintenance, and output in Drosophila Circadian clocks drive daily rhythms in metabolism, physiology, and behavior in organisms ranging from cyanobacteria to humans. The identification and analysis of “clock genes” in Drosophila revealed that circadian timekeeping is based on a transcriptional feedback loop Paul Hardin studied the development of the sea in which CLOCK-CYCLE (CLK-CYC) heterodimers activate transcription of their feedback urchin embryo in William Klein’s lab at Indiana repressors PERIOD (PER) and TIMELESS (TIM). Subsequent studies revealed that similar University, from where he received his Ph.D. in transcriptional feedback loops keep circadian time in all eukaryotes and, in the case of 1987. He did his postdoctoral fellowship with animals, that these feedback loops are comprised of conserved components. The “core” Michael Rosbash at Brandeis University, working feedback loop described above operates in conjunction with an “interlocked” feedback on the circadian rhythms of the fruit fly, Drosophila loop in animals to drive rhythmic transcription of hundreds of genes that are maximally melanogaster. His work with Michael Rosbash and expressed at different phases of the circadian cycle. These feedback loops operate in many, Jeff Hall has been instrumental to our understanding but not all, tissues in flies including the brain pacemaker neurons that control rest:activity of how circadian rhythms affect a myriad of rhythms. -

Regulation of Drosophila Circadian Rhythms by Mirna Let-7 Is Mediated by a Regulatory Cycle
ARTICLE Received 10 Jul 2014 | Accepted 10 Oct 2014 | Published 24 Nov 2014 DOI: 10.1038/ncomms6549 Regulation of Drosophila circadian rhythms by miRNA let-7 is mediated by a regulatory cycle Wenfeng Chen1,*, Zhenxing Liu1,*, Tianjiao Li1, Ruifeng Zhang1, Yongbo Xue1, Yang Zhong1, Weiwei Bai1, Dasen Zhou1 & Zhangwu Zhao1 MicroRNA-mediated post-transcriptional regulations are increasingly recognized as important components of the circadian rhythm. Here we identify microRNA let-7, part of the Drosophila let-7-Complex, as a regulator of circadian rhythms mediated by a circadian regulatory cycle. Overexpression of let-7 in clock neurons lengthens circadian period and its deletion attenuates the morning activity peak as well as molecular oscillation. Let-7 regulates the circadian rhythm via repression of CLOCKWORK ORANGE (CWO). Conversely, upregulated cwo in cwo-expressing cells can rescue the phenotype of let-7-Complex overexpression. Moreover, circadian prothoracicotropic hormone (PTTH) and CLOCK- regulated 20-OH ecdysteroid signalling contribute to the circadian expression of let-7 through the 20-OH ecdysteroid receptor. Thus, we find a regulatory cycle involving PTTH, a direct target of CLOCK, and PTTH-driven miRNA let-7. 1 Department of Entomology, College of Agronomy and Biotechnology, China Agricultural University, Beijing 100193, China. * These authors contributed equally to this work. Correspondence and requests for materials should be addressed to Z.Z. (email: [email protected]). NATURE COMMUNICATIONS | 5:5549 | DOI: 10.1038/ncomms6549 | www.nature.com/naturecommunications 1 & 2014 Macmillan Publishers Limited. All rights reserved. ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms6549 lmost all animals display a wide range of circadian bantam-dependent regulation of Clk expression is required for rhythms in behaviour and physiology, such as locomotor circadian rhythm. -
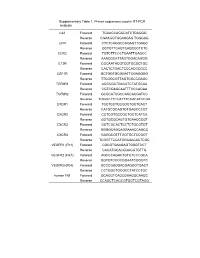
Supplementary Table 1. Primer Sequences Used in RT-PCR Analysis
Supplementary Table 1. Primer sequences used in RT-PCR analysis L32 Forward TGAAGCAGGCATCTGAGGG Reverse CGAAGGTGGAAGAG TGGGAG LIFR Forward CTCTCAGGCCAGAGTTGAGC Reverse GCTGTTCAGTCAGCCCTCTC CCR2 Forward TGTCTTCCCTGAATTGAGCC Reverse AAACGCATTAGTGGACAGGG IL12R Forward CGCAATACGTCGTGCGCTGC Reverse CACTCTGACTCCCACGCGCC CSF1R Forward GCTGGTGCGGATTCGAGGGG Reverse TTCGGCGTTAGTGGCCGAGC TGFbR1 Forward ACGCGCTGACATCTATGCAA Reverse CGTCGAGCAATTTCCCAGAA TGFbR2 Forward GCGCATCGCCAGCACGATCC Reverse TGGGCTTCCATTTCCACATCCGA CXCR1 Forward TCCTCCTGCCGCTGCTCACT Reverse CATGCGCAGTGTGAGCCCGT CXCR2 Forward CCTCGTGCCGCTGCTCATCA Reverse GGTGCGCAGTGTGAACCCGT CXCR3 Forward GGTCGCACTGCTCTGCGTGT Reverse GGGGCAGCAGGAAACCAGCC CXCR4 Forward GAGGCGTTTGGTGCTCCGGT Reverse TCGGTTCCATGGCAACACTCGC VEGFR1 (Flt1) Forward CGCGTGAAGAGTGGGTCCT Reverse CACATGCACGGAGGTGTTG VEGFR2 (Flk1) Forward AGCCCAGACTGTGTCCCGCA Reverse GGTGTCCGCGGAATCGGGTC VEGFR3 (Flt4) Forward GCCCGAGGACGAGGGTGACT Reverse CCTGGCTGCGCCTATCCTGC human Flt1 Forward GCACGTCAGCGAAGGCAAGC Reverse CCAGCTCAGCGTGGTCGTAGG Supplementary Table 2. List of putative miR-200 target genes amplified in human cancers amplified in cancers (Tumorscape)a Gene Symbol Title various cancers NSCLC PCT Errfi1 ERBB receptor feedback inhibitor 1 - yes 0.94 D11Bwg0517e DNA segment, Chr 11, Brigham & Women's Genetics 0517 expressed - yes 0.91 Pvrl4 poliovirus receptor-related 4 yes - 0.91 Mecp2 methyl CpG binding protein 2 yes yes 0.9 Jun Jun oncogene yes - 0.88 Prdm16 PR domain containing 16 - yes 0.88 Flt1 FMS-like tyrosine kinase 1 yes -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Metabolic Inactivation of the Circadian Transmitter, Pigment Dispersing Factor (PDF), by Neprilysin-Like Peptidases in Drosophila R
4465 The Journal of Experimental Biology 210, 4465-4470 Published by The Company of Biologists 2007 doi:10.1242/jeb.012088 Metabolic inactivation of the circadian transmitter, pigment dispersing factor (PDF), by neprilysin-like peptidases in Drosophila R. Elwyn Isaac1,*, Erik C. Johnson2, Neil Audsley3 and Alan D. Shirras4 1Faculty of Biological Sciences, University of Leeds, Leeds, LS2 9JT, UK, 2Department of Biology, Wake Forest University, NC, USA, 3Central Science Laboratory, Sand Hutton, York, YO41 1LZ, UK and 4Department of Biological Sciences, University of Lancaster, LA1 4YQ, UK *Author for correspondence (e-mail: [email protected]) Accepted 4 October 2007 Summary Recent studies have firmly established pigment confirming that such cleavage results in PDF inactivation. dispersing factor (PDF), a C-terminally amidated The Ser7–Leu8 peptide bond was also the principal octodecapeptide, as a key neurotransmitter regulating cleavage site when PDF was incubated with membranes rhythmic circadian locomotory behaviours in adult prepared from heads of adult Drosophila. This Drosophila melanogaster. The mechanisms by which PDF endopeptidase activity was inhibited by the neprilysin –1 functions as a circadian peptide transmitter are not fully inhibitors phosphoramidon (IC50, 0.15·mol·l ) and –1 understood, however; in particular, nothing is known thiorphan (IC50, 1.2·mol·l ). We propose that cleavage by about the role of extracellular peptidases in terminating a member of the Drosophila neprilysin family of PDF signalling at synapses. In this study we show that PDF endopeptidases is the most likely mechanism for is susceptible to hydrolysis by neprilysin, an endopeptidase inactivating synaptic PDF and that neprilysin might have that is enriched in synaptic membranes of mammals and an important role in regulating PDF signals within insects. -

Knockdown of EIF5A2 Inhibits the Malignant Potential of Non-Small Cell Lung Cancer Cells
ONCOLOGY LETTERS 15: 4541-4549, 2018 Knockdown of EIF5A2 inhibits the malignant potential of non-small cell lung cancer cells CHENG CHEN1*, BOJIA ZHANG2*, SHANSHAN WU3, YONGXIANG SONG1 and JIAN LI1 1Department of Thoracic Surgery, Affiliated Hospital of Zunyi Medical College, Zunyi, Guizhou 563000; 2Department of Infectious Disease, Shanghai 10th People's Hospital, Tongji University School of Medicine, Shanghai 200072; 3Department of Nursing, Affiliated Hospital of Zunyi Medical College, Zunyi, Guizhou 563000, P.R. China Received March 16, 2016; Accepted February 23, 2017 DOI: 10.3892/ol.2018.7832 Abstract. Eukaryotic translation initiation factor 5A2 through these proteins. EIF5A2 may therefore serve as a novel (EIF5A2) has been demonstrated to be upregulated in therapeutic target for the treatment of NSCLC. numerous types of human cancer and is associated with cancer progression. However, the expression and role of Introduction EIF5A2 in non-small cell lung cancer (NSCLC) remains unclear. In the present study, the role of EIF5A2 in NSCLC Non-small cell lung cancer (NSCLC) accounts for 85% of was investigated, in addition to the underlying molecular pulmonary malignancies (1) and is one of the leading causes of mechanisms by which EIF5A2 acts. Relative EIF5A2 expres- cancer-associated mortality worldwide. Recurrent and meta- sion levels were determined in NSCLC cells and compared static disease is the main cause of mortality, and the 5-year with levels in non-cancerous lung tissues. Short interfering survival rate for lung cancer is 15% (2). Therefore, in order to (si)RNA targeted against EIF5A2 was used to knock down improve the prognosis for patients with NSCLC, it is impor- EIF5A2 levels in NSCLC cells. -

Pololu Jrk USB Motor Controller User's Guide
Pololu Jrk USB Motor Controller User’s Guide © 2001–2019 Pololu Corporation Pololu Jrk USB Motor Controller User’s Guide https://www.pololu.com/docs/0J38/all Page 1 of 55 Pololu Jrk USB Motor Controller User’s Guide © 2001–2019 Pololu Corporation 1. Overview . 3 1.a. Module Pinout and Components . 6 1.b. Supported Operating Systems . 9 1.c. PID Calculation Overview . 10 2. Contacting Pololu . 12 3. Configuring the Motor Controller . 13 3.a. Installing Windows Drivers and the Configuration Utility . 13 3.b. Input Options . 18 3.c. Feedback Options . 20 3.d. PID Options . 22 3.e. Motor Options . 24 3.f. Error Response Options . 27 3.g. The Plots Window . 29 3.h. Upgrading Firmware . 30 4. Using the Serial Interface . 33 4.a. Serial Modes . 33 4.b. TTL Serial . 34 4.c. Command Protocols . 36 4.d. Cyclic Redundancy Check (CRC) Error Detection . 37 4.e. Motor Control Commands . 39 4.f. Error Reporting Commands . 41 4.g. Variable Reading Commands . 44 4.h. Daisy-Chaining . 46 4.i. Serial Example Code . 48 4.i.1. Cross-platform C . 48 4.i.2. Windows C . 50 5. Setting Up Your System . 51 6. Writing PC Software to Control the Jrk . 55 Page 2 of 55 Pololu Jrk USB Motor Controller User’s Guide © 2001–2019 Pololu Corporation 1. Overview The jrk family of versatile, general-purpose motor controllers supports a variety of interfaces, including USB. Analog voltage and tachometer (frequency) feedback options allow quick implementation of closed-loop servo systems, and a free configuration utility (for Windows) allows easy calibration and configuration through the USB port. -

EIF5A2 Controls Ovarian Tumor Growth and Metastasis by Promoting Epithelial to Mesenchymal Transition Via the Title Tgfβ Pathway
EIF5A2 controls ovarian tumor growth and metastasis by promoting epithelial to mesenchymal transition via the Title TGFβ pathway Zhao, Guannan; Zhang, Wenjing; Dong, Peixin; Watari, Hidemichi; Guo, Yuqi; Pfeffer, Lawrence M; Tigyi, Gabor; Author(s) Yue, Junming Cell & Bioscience, 11, 70 Citation https://doi.org/10.1186/s13578-021-00578-5 Issue Date 2021-04-07 Doc URL http://hdl.handle.net/2115/80967 Rights(URL) https://creativecommons.org/licenses/by/4.0/ Type article File Information Yue EIF5A2 paper.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Zhao et al. Cell Biosci (2021) 11:70 https://doi.org/10.1186/s13578-021-00578-5 Cell & Bioscience RESEARCH Open Access EIF5A2 controls ovarian tumor growth and metastasis by promoting epithelial to mesenchymal transition via the TGFβ pathway Guannan Zhao1,2, Wenjing Zhang3, Peixin Dong4, Hidemichi Watari4, Yuqi Guo5*, Lawrence M. Pfefer1,2, Gabor Tigyi6 and Junming Yue1,2* Abstract Background: Epithelial to mesenchymal transition (EMT) contributes to tumor metastasis and chemoresistance. Eukaryotic initiation factor 5A2 (EIF5A2) is highly expressed in a variety of human cancers but rarely expressed in normal tissues. While EIF5A2 has oncogenic activity in several cancers and contributes to tumor metastasis, its role in ovarian cancer is unknown. In this study, we investigate whether EIF5A2 contributes to ovarian tumor metastasis by promoting EMT. Methods: To investigate the role of EIF5A2, we knocked out (KO) EIF5A2 using lentiviral CRISPR/Cas9 nickase in high invasive SKOV3 and OVCAR8 cells and overexpressed EIF5A2 in low invasive OVCAR3 cells using lentiviral vector. Cell proliferation, migration and invasion was examined in vitro ovarian cancer cells and tumor metastasis was evaluated in vivo using orthotopic ovarian cancer mouse models. -

WO 2019/079361 Al 25 April 2019 (25.04.2019) W 1P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2019/079361 Al 25 April 2019 (25.04.2019) W 1P O PCT (51) International Patent Classification: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, C12Q 1/68 (2018.01) A61P 31/18 (2006.01) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, C12Q 1/70 (2006.01) HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, (21) International Application Number: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, PCT/US2018/056167 OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (22) International Filing Date: SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 16 October 2018 (16. 10.2018) TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (26) Publication Language: English GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, (30) Priority Data: UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, 62/573,025 16 October 2017 (16. 10.2017) US TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, ΓΕ , IS, IT, LT, LU, LV, (71) Applicant: MASSACHUSETTS INSTITUTE OF MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, TECHNOLOGY [US/US]; 77 Massachusetts Avenue, TR), OAPI (BF, BJ, CF, CG, CI, CM, GA, GN, GQ, GW, Cambridge, Massachusetts 02139 (US). -

PDF Cycling in the Dorsal Protocerebrum of the Drosophila Brain Is Not Necessary for Circadian Clock Function
PDF Cycling in the Dorsal Protocerebrum of the Drosophila Brain Is Not Necessary for Circadian Clock Function Elzbieta Kula,* Edwin S. Levitan,† Elzbieta Pyza,‡ and Michael Rosbash*,1 *Department of Biology-HHMI, Brandeis University, Waltham, MA, the †Department of Pharmacology, University of Pittsburgh, Pittsburgh, Pennsylvania, and the ‡Department of Cytology and Histology, Institute of Zoology, Jagiellonian University, Krakow, Poland Abstract In Drosophila, the neuropeptide pigment-dispersing factor (PDF) is a likely circadian molecule, secreted by central pacemaker neurons (LNvs). PDF is expressed in both small and large LNvs (sLNvs and lLNvs), and there are striking circadian oscillations of PDF staining intensity in the small cell termini, which require a functional molecular clock. This cycling may be relevant to the proposed role of PDF as a synchronizer of the clock system or as an output signal connect- ing pacemaker cells to locomotor activity centers. In this study, the authors use a generic neuropeptide fusion protein (atrial natriuretic factor–green fluorescent protein [ANF-GFP]) and show that it can be expressed in the same neurons as PDF itself. Yet, ANF-GFP as well as PDF itself does not manifest any cyclical accumu- lation in sLNv termini in adult transgenic flies. Surprisingly, the absence of detectable PDF cycling is not accompanied by any detectable behavioral pheno- type, since these transgenic flies have normal morning and evening anticipation in a light-dark cycle (LD) and are fully rhythmic in constant darkness (DD). The molecular clock is also not compromised. The results suggest that robust PDF cycling in sLNv termini plays no more than a minor role in the Drosophila circa- dian system and is apparently not even necessary for clock output function. -

Translational Control in Cancer Etiology
Downloaded from http://cshperspectives.cshlp.org/ on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press Translational Control in Cancer Etiology Davide Ruggero Helen Diller Cancer Center, School of Medicine, University of California, San Francisco, California 94158 Correspondence: [email protected] The link between perturbations in translational control and cancer etiology is becoming a primary focus in cancer research. It has now been established that genetic alterations in several components of the translational apparatus underlie spontaneous cancers as well as an entire class of inherited syndromes known as “ribosomopathies” associated with in- creased cancer susceptibility. These discoveries have illuminated the importance of dereg- ulations in translational control to very specific cellular processes that contribute to cancer etiology. In addition, a growing body of evidence supports the view that deregulation of translational control is a common mechanism by which diverse oncogenic pathways promote cellular transformation and tumor development. Indeed, activation of these key oncogenic pathways induces rapid and dramatic translational reprogramming both by in- creasing overall protein synthesis and by modulating specific mRNA networks. These trans- lational changes promote cellular transformation, impacting almost every phase of tumor development. This paradigm represents a new frontier in the multihit model of cancer for- mation and offers significant promise for innovative cancer therapies. Current research,